- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Periodic table of elements
Содержание
- 2. Co
- 3. history of discovery1735Swedish mineralogistGeorg Brandt
- 4. physical propertieshard metalexists in two versionsmelting point of 1494 ° Cferromagnetic material
- 5. chemical properties2Co + O2 = 2CoOCo +
- 6. biological functionVital for the body trace element.It
- 7. Ni
- 8. history of discovery1751swedish mineralogistCronstedt
- 9. physical propertiessilver-white metaldoes not tarnish in airHas
- 10. chemical properties2Ni + O2 = 2NiO3Ni +
- 11. biological functionIt is one of trace elements
- 12. Hf
- 13. history of discovery1923French chemistJean UrbainDanish chemist:Dirk Coster and Georg de Hevesy
- 14. physical propertiessilvery-white, having a surface with a
- 15. chemical propertiesHf + 2F2 = HfF4Other reactions
- 16. biological functionnot installed
- 17. He
- 18. history of discoveryAugust 18, 1868French scientistPierre JanssenOctober 20, 1868English astronomerNorman Lockyer
- 19. physical propertiespractically inert chemical element.nontoxicis colorless, odorless
- 20. chemical propertiesinert gas
- 21. biological functionAt the moment, the biological role is not clear
- 22. Скачать презентацию
- 23. Похожие презентации
history of discovery1735Swedish mineralogistGeorg Brandt





















Слайд 5
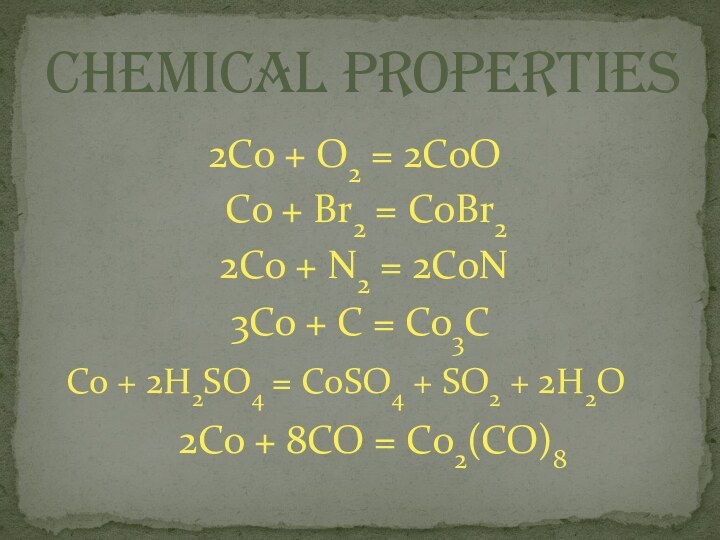
chemical properties
2Co + O2 = 2CoO
Co + Br2
= CoBr2
2Co + N2 = 2CoN
3Co + C =
Co3CCo + 2H2SO4 = CoSO4 + SO2 + 2H2O
2Co + 8CO = Co2(CO)8
Слайд 6
biological function
Vital for the body trace element.
It is
a part of vitamin B12 (cobalamin).
It is involved in
blood formation, function of the nervous system and liver, enzymatic reactions.The human need for cobalt 0,007-0,015 mg daily.
In the absence of cobalt akobaltoz develops.
Слайд 9
physical properties
silver-white metal
does not tarnish in air
Has a
face-centered cubic lattice
In its pure form is very plastic
and easy to work pressure.Density (at n. Y.) = 8.902 g / cm ³
Melting point = 1726 K
Слайд 10
chemical properties
2Ni + O2 = 2NiO
3Ni + N2
= Ni3N2
2Ni + B = Ni2B
Ni + 4HNO3 =
Ni(NO3)2 + 2NO2 + 2H2ONi + CuSO4 = NiSO4 + Cu
Ni + 4CO = Ni(CO)4
Ni + Cl2 = NiCl2
Слайд 11
biological function
It is one of trace elements necessary
for the normal development of living organisms.
It takes part
in enzymatic reactions in animals and plants.In the body, it accumulates in animal dead skin tissues, especially in the feathers.
Слайд 13
history of discovery
1923
French chemist
Jean Urbain
Danish chemist:
Dirk Coster and
Georg de Hevesy
Слайд 14
physical properties
silvery-white, having a surface with a bright
luster is not fading
At ordinary temperatures it has a
hexagonal latticeDensity = 13.09 g/cm3 (20 ° C)
refractory metal, its melting temperature = 2222 ° C
One of the rare natural isotopes of hafnium, 174Hf, exhibits a weak alpha activity (half-life of 2.1015 years)