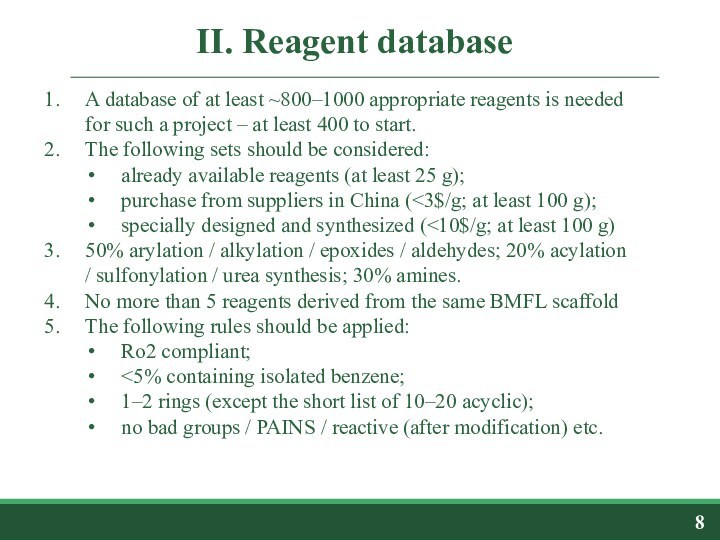
scaffold library
II. Reshaping of the reagent library
IV. Two-phase
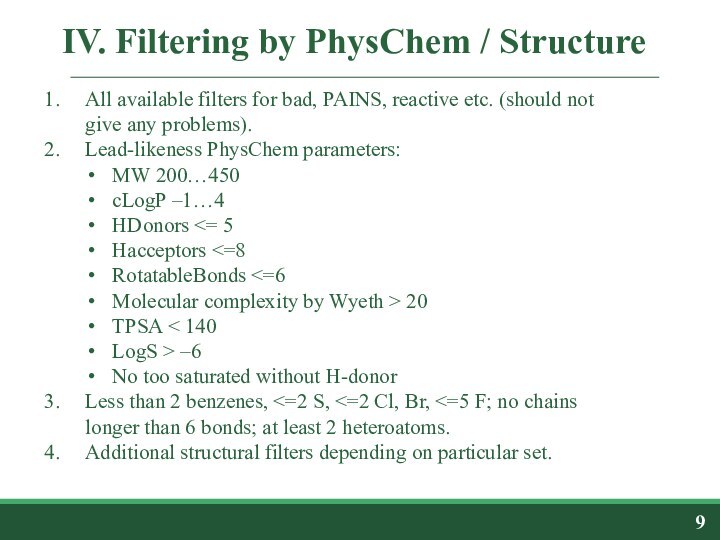
diversity selectionIV. Filtering by physico-chemical/structural parameters
V. Checking for the sufficient novelty
III. Virtual coupling
VI. Control of Fsp3 and abundant chemotypes