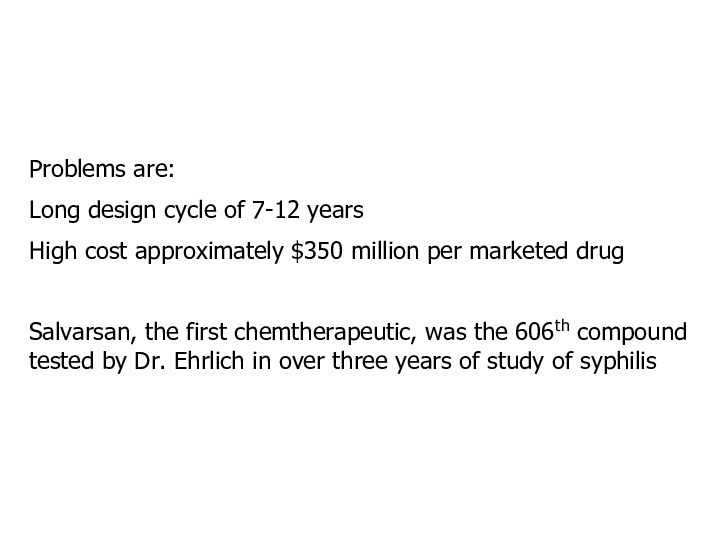Слайд 3
How are drugs created or discovered?
Natural drug products
have been used for millenia
Synthetic drugs came into being
during the 19th century
Today, drugs are still come from this two sources
Chemicals found in nature or synthesized in labs are randomly screened for their therapeutic ability
Слайд 4
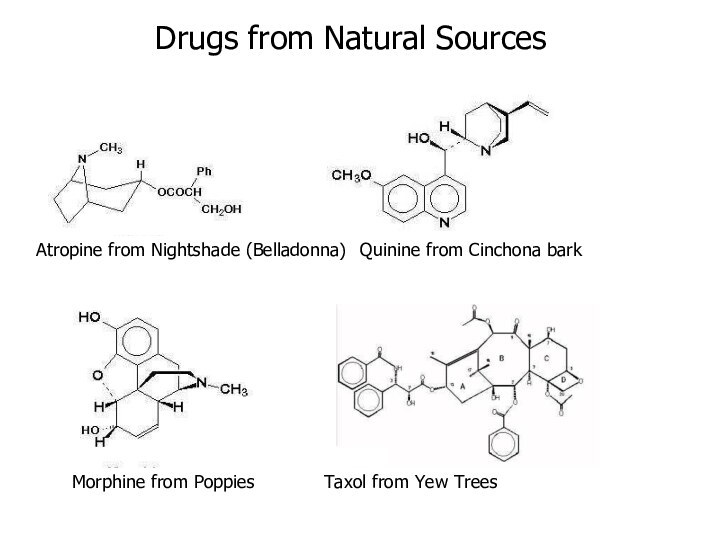
Atropine from Nightshade (Belladonna) Quinine from Cinchona bark
Morphine from
Poppies Taxol from Yew Trees
Drugs from Natural Sources
Слайд 5
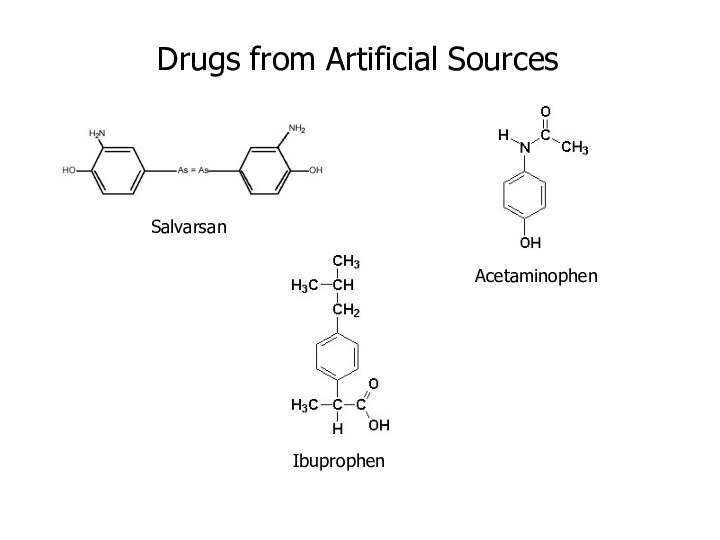
Salvarsan
Drugs from Artificial Sources
Acetaminophen
Ibuprophen
Слайд 6
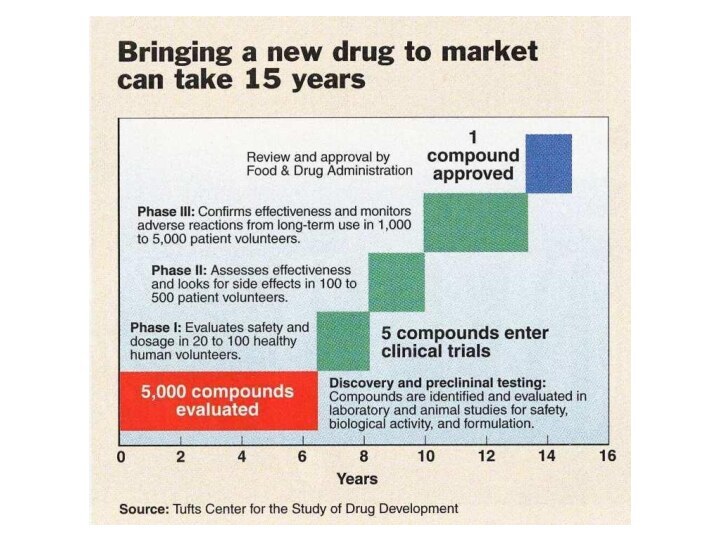
Problems are:
Long design cycle of 7-12 years
High cost
approximately $350 million per marketed drug
Salvarsan, the first chemtherapeutic,
was the 606th compound tested by Dr. Ehrlich in over three years of study of syphilis
Слайд 7
One way to increase the odds of finding
a drug is through High Throughput Screening (HTS)
HTS seeks
to increase the number of compounds tested at one time for drug-like properties
By testing 100s to 1000s of compounds at one time, HTS allows a drug company to search through many compounds in less time
Potential compounds are screened using plates capable of holding 96 to over 3000 different compounds
Слайд 8
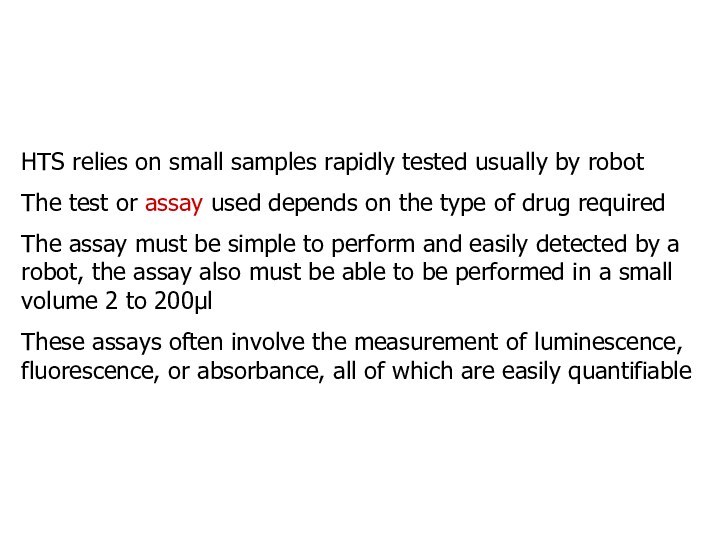
HTS relies on small samples rapidly tested usually
by robot
The test or assay used depends on the
type of drug required
The assay must be simple to perform and easily detected by a robot, the assay also must be able to be performed in a small volume 2 to 200l
These assays often involve the measurement of luminescence, fluorescence, or absorbance, all of which are easily quantifiable
Слайд 9
What are the targets of the drugs developed
or what do they screen against?
Traditionally drugs were first
tested against an animal or a human who had the disease the company is interested in creating a drug against
This is expensive, time consuming & can be dangerous
While this is still done, it is done at a much later stage in the drug development
Some HTS assays use cells, but many are cell-free or in vitro
There are some HTS assays though that use organisms, but these are mainly flies, worms, or fish
Слайд 10
http://www.pcop.com/dd/techno/tech_hts.html
96, 384, & 1536 well plates
Hold 100,
20, 2 l/well respectively
Assay Plates
Слайд 11
3456-well plate, each well holds 200nl
Слайд 12
http://www.noabbiodiscoveries.com/hts.htm
Robot pipetting samples into a 96 well plate
Слайд 13
http://mango2.vtt.fi:84/bel/services/hts.htm
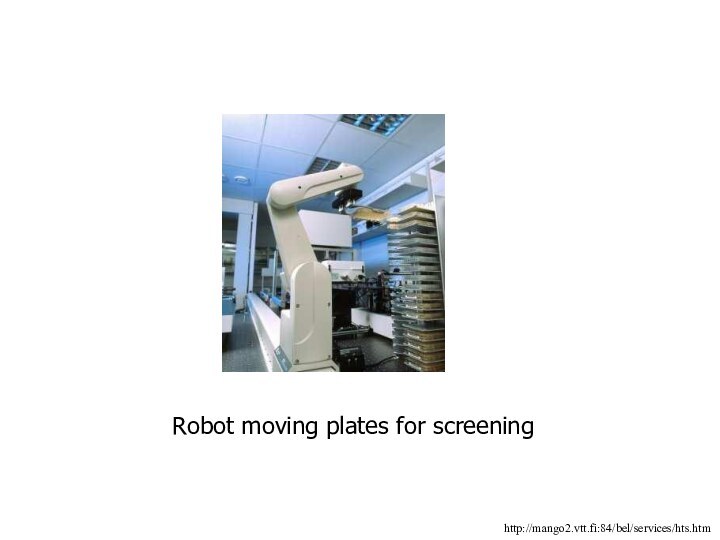
Robot moving plates for screening
Слайд 14
http://www.thomasnewman.com/novartis/public/helping/txt_02.html
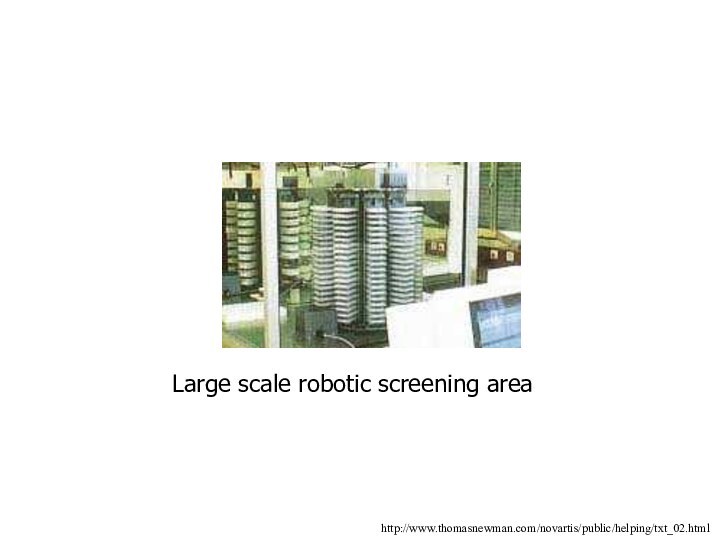
Large scale robotic screening area
Слайд 15
Zebrafish in the well of a 96-well plate
Слайд 16
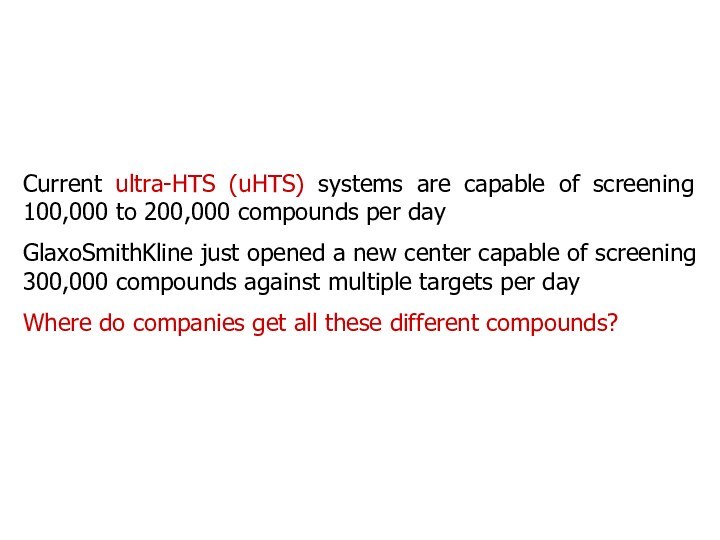
Current ultra-HTS (uHTS) systems are capable of screening
100,000 to 200,000 compounds per day
GlaxoSmithKline just opened a
new center capable of screening 300,000 compounds against multiple targets per day
Where do companies get all these different compounds?
Слайд 18
Combinatorial libraries are large collections of randomly generated
compounds usually based on a scaffold molecule
The scaffold molecule
often is the skeleton of a known class of drugs or a random chemical structure
The scaffold molecule is modified by the addition of functional groups such as methyl, ethyl, amino, or carboxyl groups
Libraries can contain anywhere from 500 to 50,000 randomly generated members
These libraries are then screened for possible drug compounds
Слайд 19
http://schultz.scripps.edu/Research/FunctionalGenomics/research.html
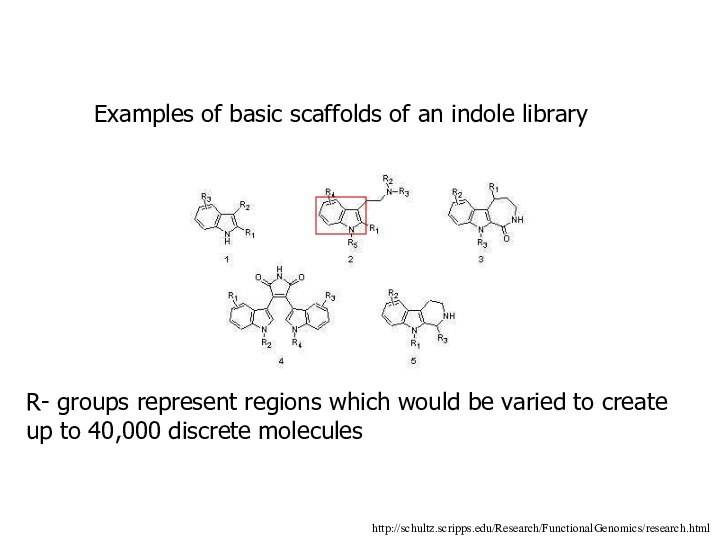
Examples of basic scaffolds of an indole library
R-
groups represent regions which would be varied to create
up to 40,000 discrete molecules
Слайд 20
Libraries are screened to find hits
Hits are active
samples that meet a defined success criteria
These criteria are
determined by the company and are specific to the assay being used
Once these hits are validated, meaning the compounds nature is confirmed, they progress to lead compound status
A lead compound is a hit with sufficient potential to progress to full drug development
Слайд 21
The lead compound then progresses to the next
phase of drug development
Where other aspects of its physical
nature are tested
The compound is assayed for toxicity, often this is done during HTS, but further tests are required in cells or whole organisms
This is also when it will be determined how the drug is to be delivered
Слайд 22
It was originally thought that combination of chemistry,
robotics, & computers would deliver blockbuster drugs
However, HTS of
random compounds has not delivered a large number of new blockbuster drugs
Companies are now taking known drugs or compounds that have drug-like properties & using these as scaffolds to create libraries
These libraries are more focused in that they are tailored to the disease being targeted
Another option is rational or structure based drug design
Слайд 23
Rational Drug Design
Engineering of a molecule or protein
through specific changes such that it becomes drug-like
Often requires
choosing a target molecule in the cell, such as a receptor or enzyme and designing a therapeutic that prevents the target from causing or contributing to a disease
Need to know the structure of the target usually obtained through X-ray crystallography or NMR
Also need a complete understanding of the thermodynamics factors involved in binding, which vary from interaction to interaction
Слайд 24
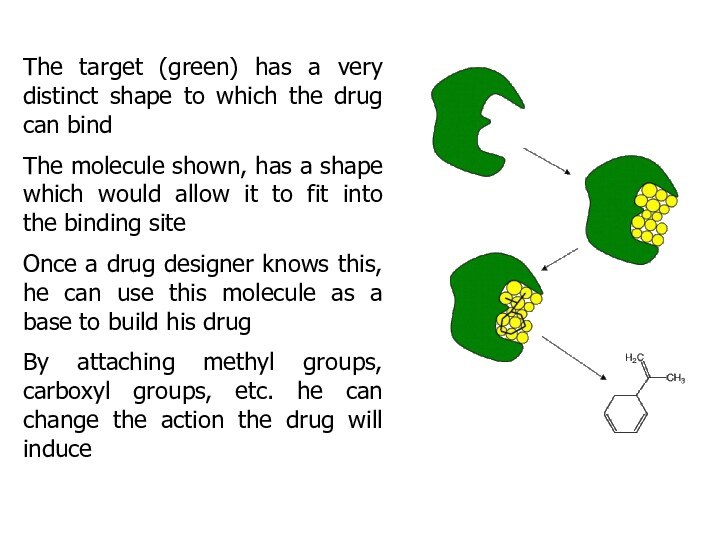
The target (green) has a very distinct shape
to which the drug can bind
The molecule shown,
has a shape which would allow it to fit into the binding site
Once a drug designer knows this, he can use this molecule as a base to build his drug
By attaching methyl groups, carboxyl groups, etc. he can change the action the drug will induce
Слайд 25
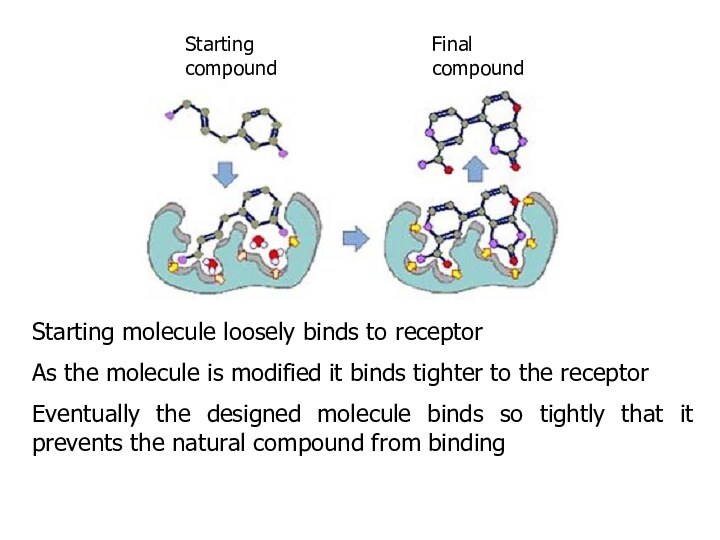
Starting molecule loosely binds to receptor
As the molecule
is modified it binds tighter to the receptor
Eventually the
designed molecule binds so tightly that it prevents the natural compound from binding
Starting compound
Final compound
Слайд 26
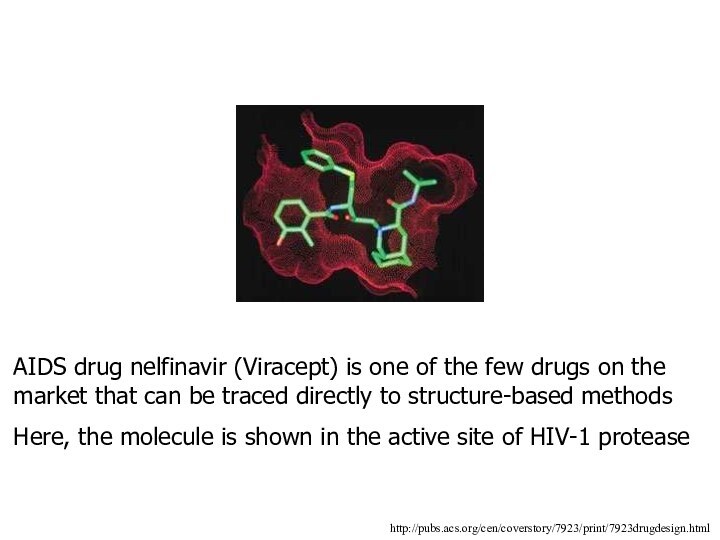
AIDS drug nelfinavir (Viracept) is one of the
few drugs on the market that can be traced
directly to structure-based methods
Here, the molecule is shown in the active site of HIV-1 protease
http://pubs.acs.org/cen/coverstory/7923/print/7923drugdesign.html
Слайд 27
Other methods of drug design are based on
taking known drugs & modifying their structure to make
them better
This requires one to know the structure of the drug
Alterations may:
Cause the drug to be more potent
Give the drug fewer side effects
Increase its solubility, giving better absorption
Слайд 28
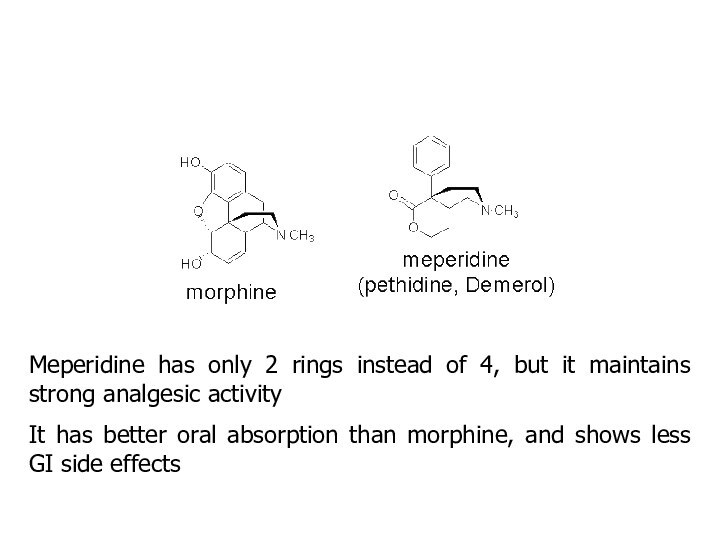
Meperidine has only 2 rings instead of 4,
but it maintains strong analgesic activity
It has better oral
absorption than morphine, and shows less GI side effects
Слайд 29
Another method of drug design is to take
a known molecule & design a drug mimic
A mimic
looks like the endogenous molecule, but is not processed by the cell the same way
These mimics work either as antagonists, that prevent cell functions
Or agonists that turn on cellular function in the absence of the normal signal
Слайд 30
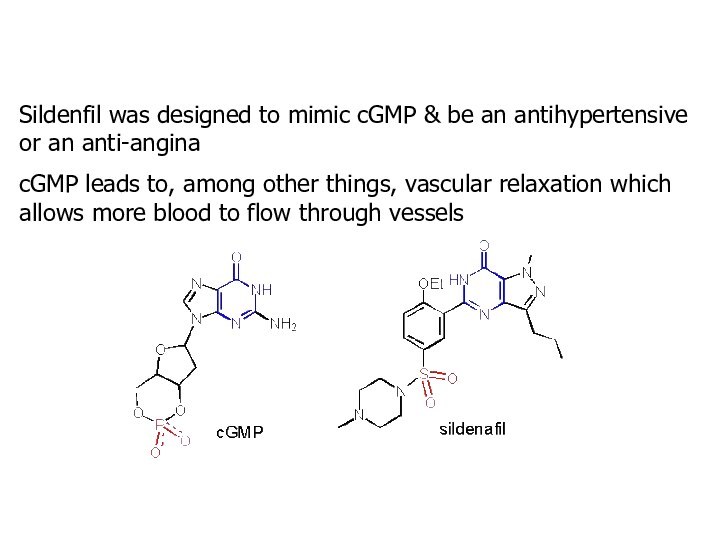
Sildenfil was designed to mimic cGMP & be
an antihypertensive or an anti-angina
cGMP leads to, among other
things, vascular relaxation which allows more blood to flow through vessels
Слайд 31
Phosphodiesterase (PDE), is the enzyme that converts cGMP
to GMP
By blocking PDE-5, sildenafil prevents the breakdown of
cGMP
Leading to more blood in the vessels
Unfortunately sildenafil did not work as well as the normal treatment, nitroglycerine
But its side effect was much more promising…
Слайд 32
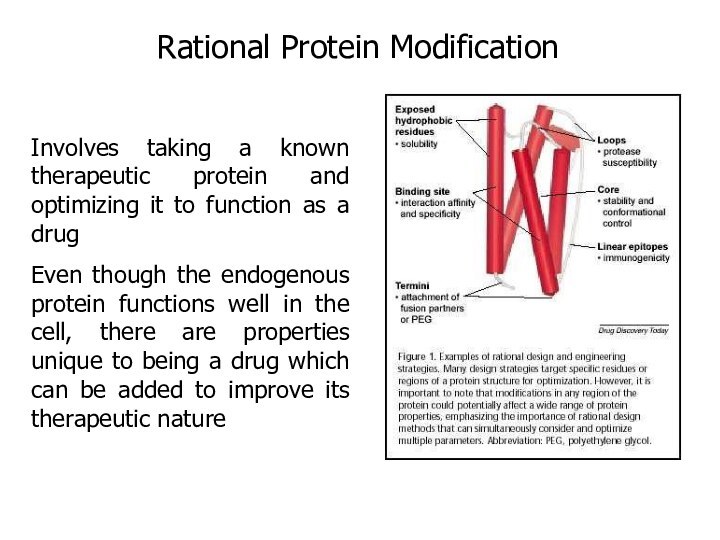
Rational Protein Modification
Involves taking a known therapeutic protein
and optimizing it to function as a drug
Even though
the endogenous protein functions well in the cell, there are properties unique to being a drug which can be added to improve its therapeutic nature