- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Basics of thermodynamics & kinetics
Содержание
- 2. THERMODYNAMISC&STATISTICAL PHYSICS
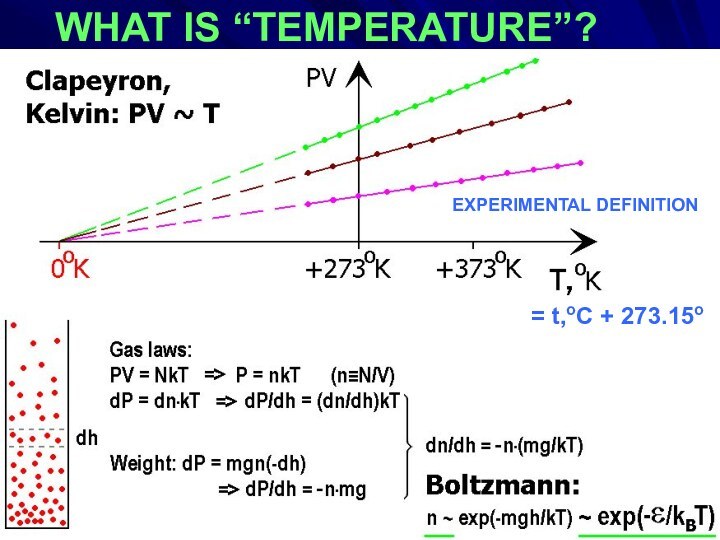
- 3. WHAT IS “TEMPERATURE”?EXPERIMENTAL DEFINITION := t,oC + 273.15oEXPERIMENTAL DEFINITION
- 4. Benoît Paul Émile Clapeyron (1799 – 1864)William
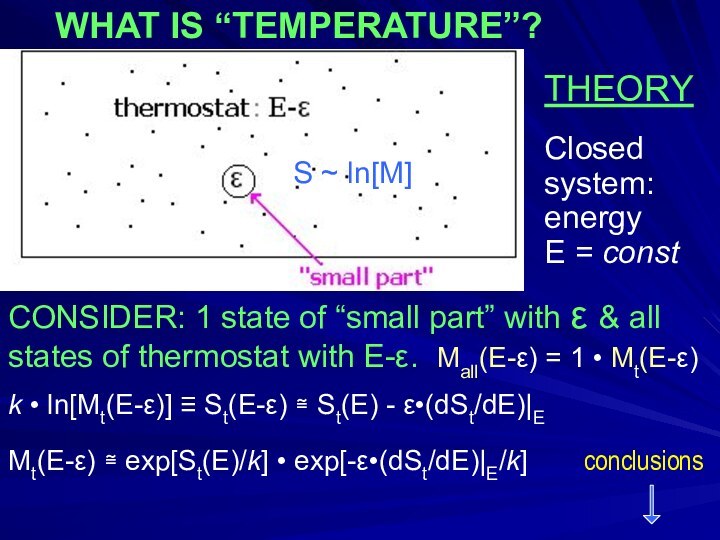
- 5. THEORYClosedsystem:energy E = constCONSIDER: 1 state
- 6. COMPARE:Probability1(ε1) = Mt(E-ε1) / M(E) =
- 7. Josiah Willard Gibbs (1839 –1903)
- 8. (dSth/dE) = 1/ TP1(ε1) ~ exp(-ε1/kBT)Pj(εj) =
- 9. Unstable (explodes, v → inf.)
- 10. Separation of potential and kinetic energiesin classic
- 11. IN THERMAL EQUILIBRIUM:TCOORD = TKIN = TouterWe
- 12. TRANSITIONS:THERMODYNAMICS
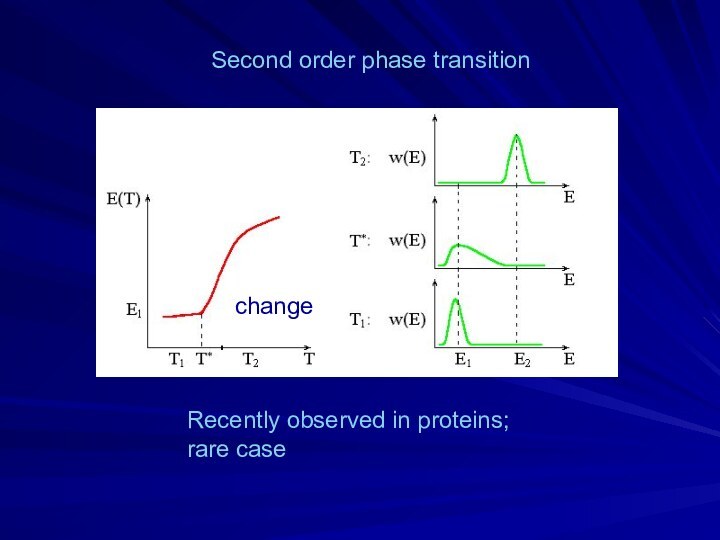
- 13. gradual transition“all-or-none” (or 1st order) phase transitioncoexistence& jump-liketransitioncoexistence(ΔE/kT*)(ΔT/T*) ~ 1Transition: |ΔF1|= |-ΔS×ΔT| ~ kT*ΔE-T*ΔS=0
- 14. Second order phase transitionchangeRecently observed in proteins;rare case
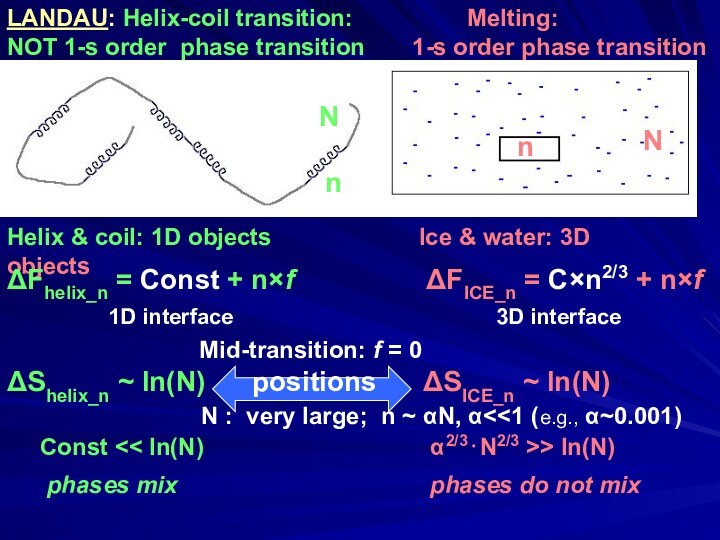
- 15. LANDAU: Helix-coil transition:
- 16. Лев Давидович Ландау (1908 - 1968)Нобелевская Премия 1962
- 17. TRANSITIONS:KINETICS
- 18. n# = n × exp(-ΔF#/kBT)n# n→TRANSITION TIME:t0→1
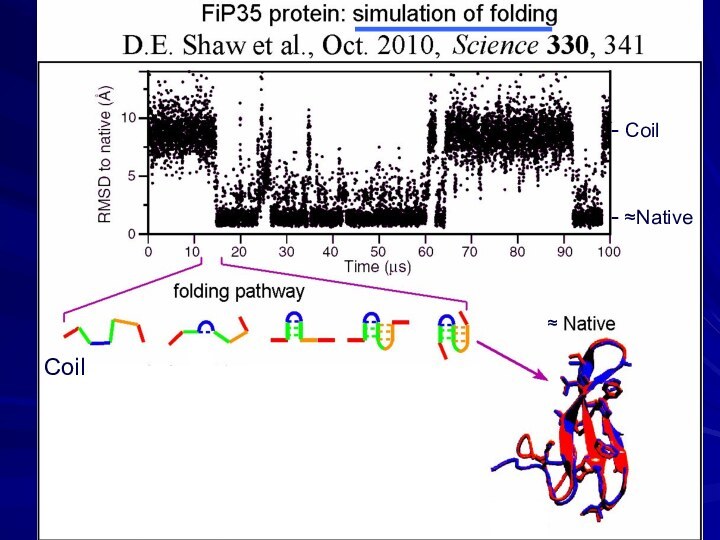
- 20. phase separationCoil- Coil- ≈Native≈
- 21. TRANSITION RATE = SUM OF RATES
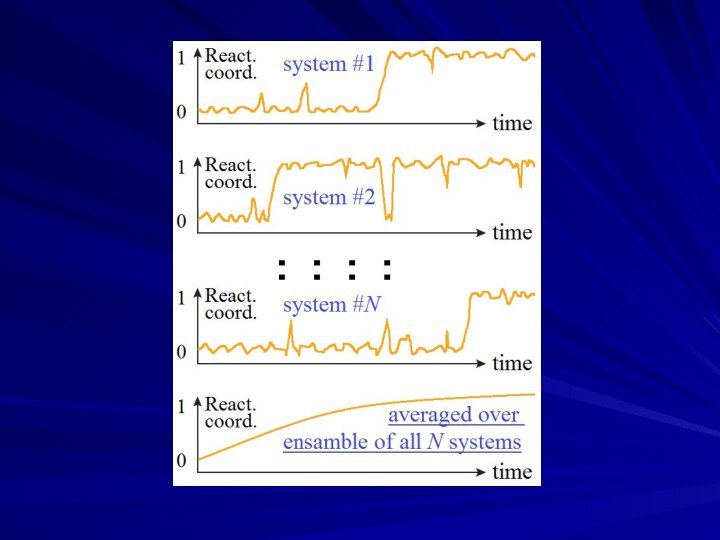
- 22. t0→… →finish ≈ t0→#1→ finish + t0→#2→
- 23. __TRANSITION TIME IS ESSENTIALLY EQUAL FOR “TRAPS”
- 24. DIFFUSION:KINETICS
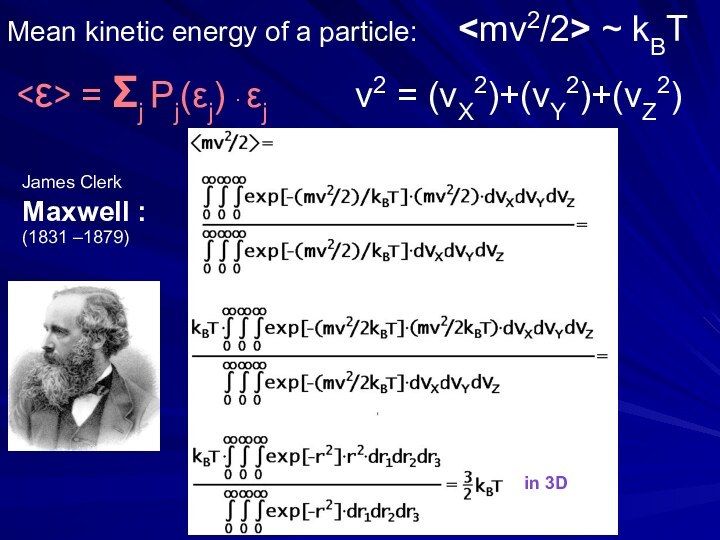
- 25. Mean kinetic energy of a particle:
- 26. Friction stops a molecule within picoseconds: m(dv/dt)
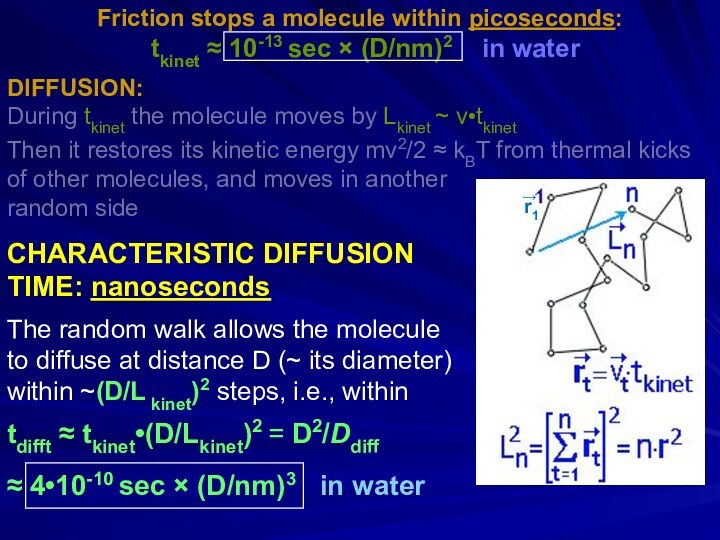
- 27. Friction stops a molecule within picoseconds:
- 28. The End
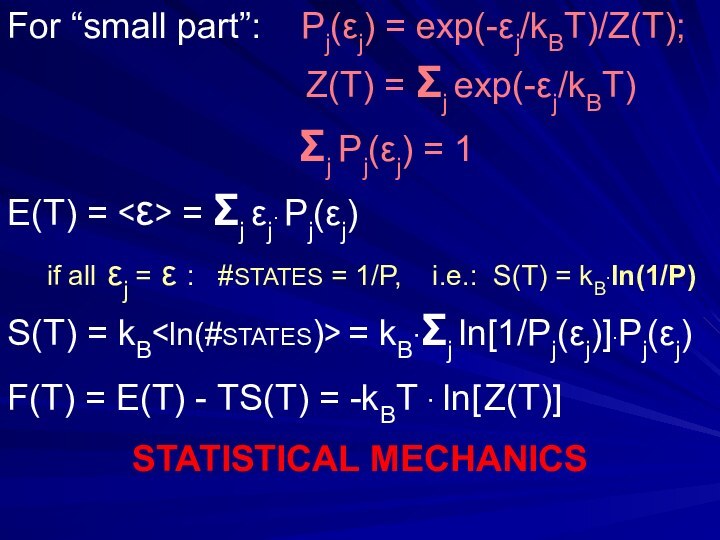
- 29. For “small part”: Pj(εj) = exp(-εj/kBT)/Z(T);
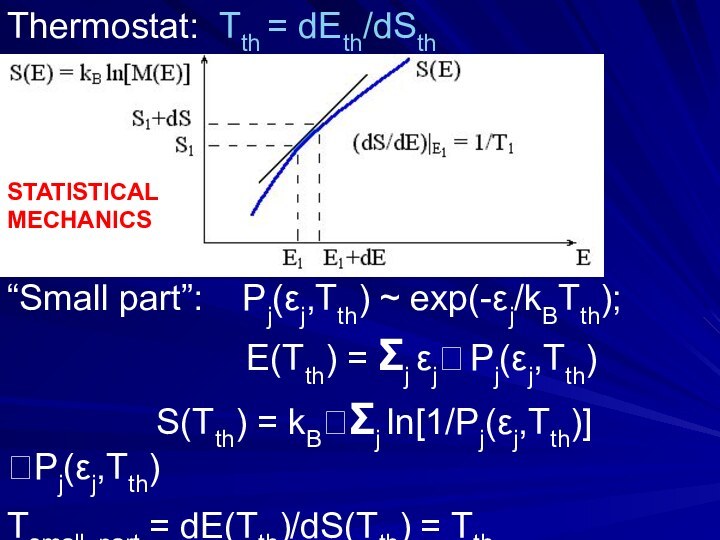
- 30. Thermostat: Tth = dEth/dSth“Small part”: Pj(εj,Tth)
- 31. Along tangent: S-S(E1) =
- 32. Separation of potential energyin classic (non-quantum) mechanics:P(ε)
- 33. P(εKIN+εCOORD) ~ exp(-εCOORD/kBT)•exp(-εKIN/kBT)P(εCOORD) = exp(-εCOORD/kBT) / ZCOORD(T)ZCOORD(T)
- 34. Скачать презентацию
- 35. Похожие презентации








![Basics of thermodynamics & kinetics Friction stops a molecule within picoseconds: m(dv/dt) = -(3πDη)v [Stokes law],](/img/tmb/15/1403069/a05044bbfa42a8508e2ed0538c453064-720x.jpg)

Слайд 4
Benoît Paul Émile Clapeyron (1799 – 1864)
William Thomson,
1st Baron Kelvin (1824 -1907)
Ludwig Eduard Boltzmann (1844 –
1906)
Слайд 5
THEORY
Closed
system:
energy
E = const
CONSIDER: 1 state of
“small part” with ε & all
states of thermostat
with E-ε. Mall(E-ε) = 1 • Mt(E-ε) k • ln[Mt(E-ε)] ≡ St(E-ε) ≅ St(E) - ε•(dSt/dE)|E
Mt(E-ε) ≅ exp[St(E)/k] • exp[-ε•(dSt/dE)|E/k] conclusions
WHAT IS “TEMPERATURE”?
S ~ ln[M]
Слайд 6
COMPARE:
Probability1(ε1) = Mt(E-ε1) / M(E) =
exp[-
ε1• (dSt/dE)|E/k] (GIBBS)and
Probability1(ε1) = exp(-ε1/kBT) (BOLTZMANN)
One has: (dSt/dE)|E = 1/ T
k = kB
______________________________________________________________
ε ⇒ ε-kBT, M ⇒ M × exp(1) ≡ M × 2.72
Слайд 7
Josiah Willard Gibbs
(1839 –1903)
Яков Григорьевич Синай, 1935
Abel Prize 2014“…связь между порядком и хаосом…” 1/r3
Joseph Liouville
(1809 - 1882)
Слайд 8
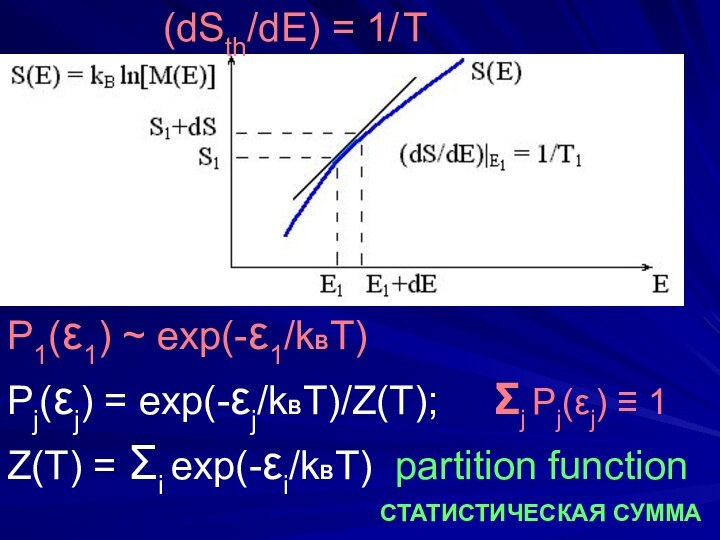
(dSth/dE) = 1/ T
P1(ε1) ~ exp(-ε1/kBT)
Pj(εj) = exp(-εj/kBT)/Z(T);
Σj Pj(εj) ≡ 1
Z(T) = Σi exp(-εi/kBT)
partition functionСТАТИСТИЧЕСКАЯ СУММА
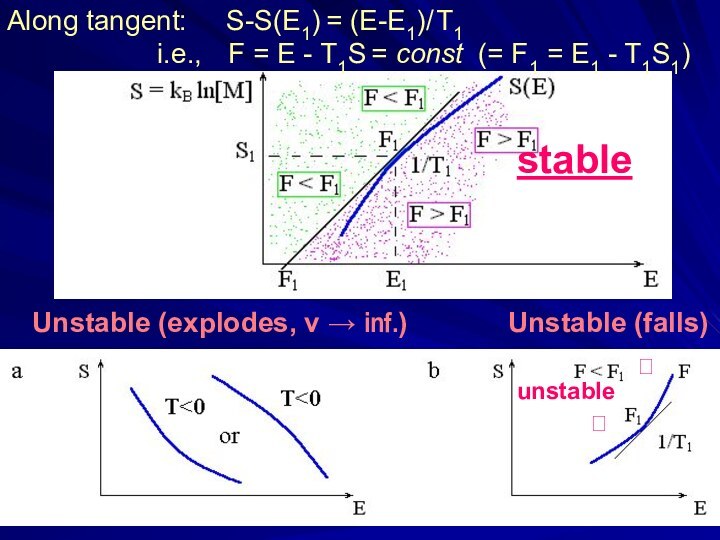
Слайд 9 Unstable (explodes, v → inf.)
Unstable (falls)
stable
?unstable
?
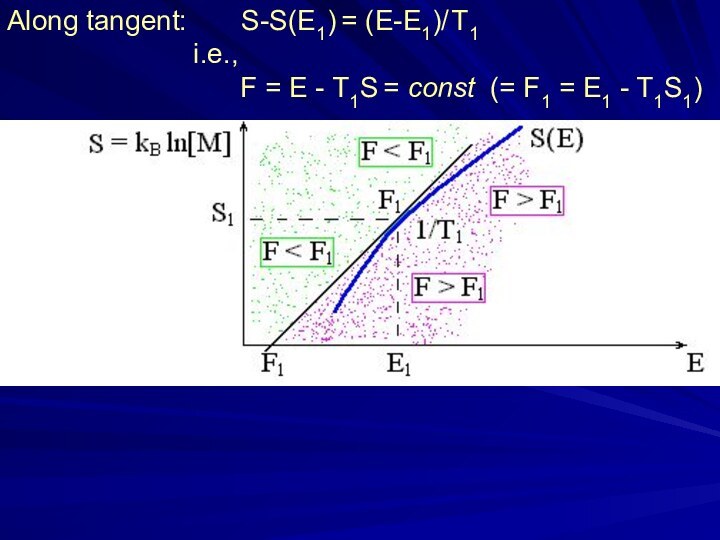
Along tangent: S-S(E1) = (E-E1)/ T1
i.e., F = E - T1S = const (= F1 = E1 - T1S1)
Слайд 10
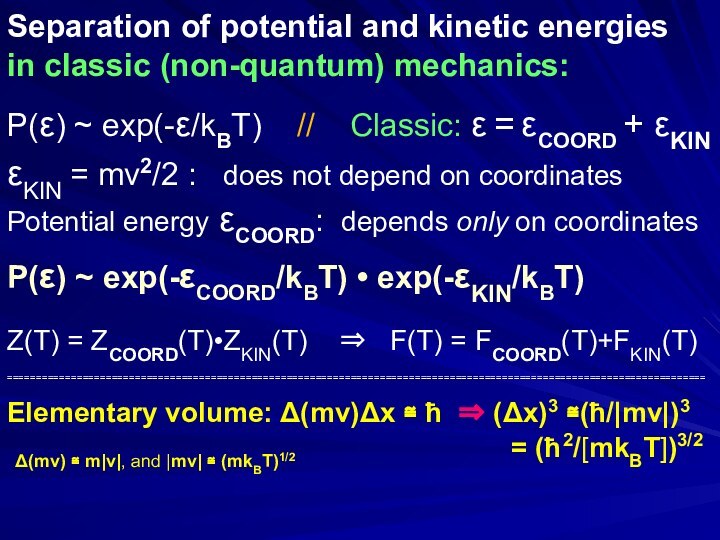
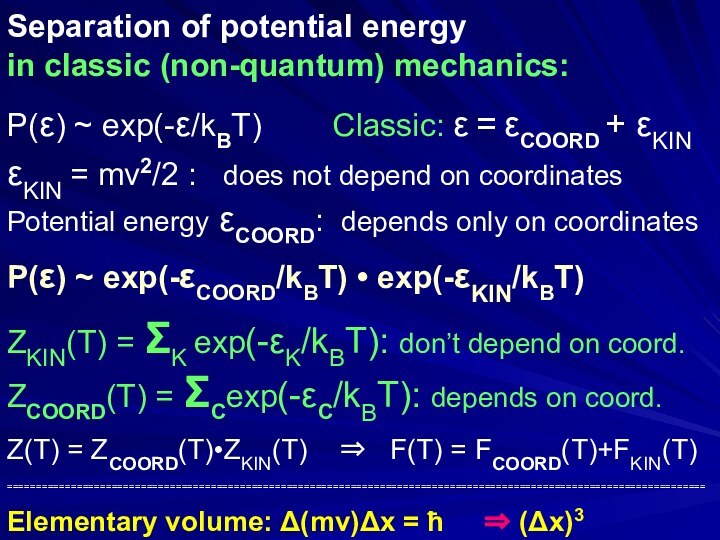
Separation of potential and kinetic energies
in classic (non-quantum)
mechanics:
P(ε) ~ exp(-ε/kBT) // Classic: ε =
εCOORD + εKIN εKIN = mv2/2 : does not depend on coordinates
Potential energy εCOORD: depends only on coordinates
P(ε) ~ exp(-εCOORD/kBT) • exp(-εKIN/kBT)
Z(T) = ZCOORD(T)•ZKIN(T) ⇒ F(T) = FCOORD(T)+FKIN(T)
========================================================================================================================
Elementary volume: Δ(mv)Δx ≅ ħ ⇒ (Δx)3 ≅(ħ/|mv|)3
= (ħ2/[mkBT])3/2
Δ(mv) ≅ m|v|, and |mv| ≅ (mkBT)1/2
Слайд 11
IN THERMAL EQUILIBRIUM:
TCOORD = TKIN = Touter
We may
consider further
only potential energy:
E ⇒ ECOORD
M ⇒ MCOORD
S(E) ⇒
SCOORD(ECOORD )F(E) ⇒ FCOORD , etc.
Слайд 13
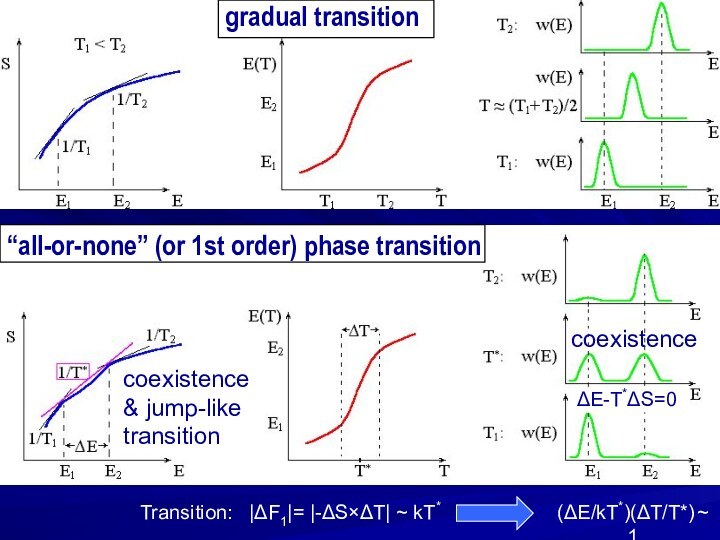
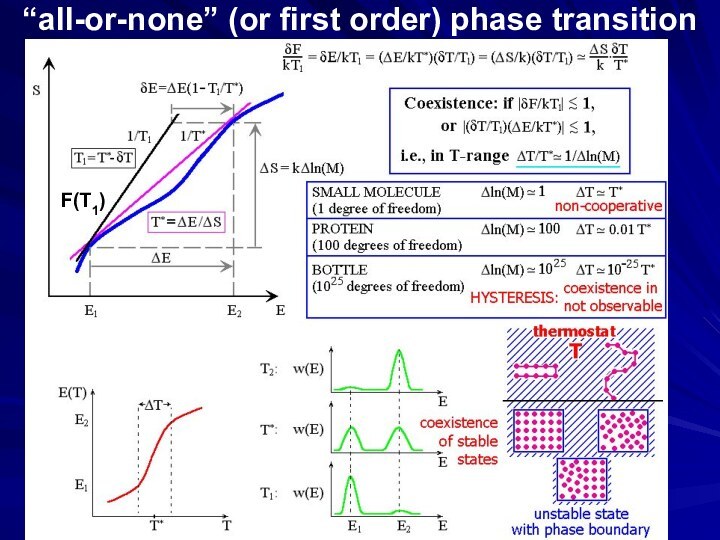
gradual transition
“all-or-none” (or 1st order) phase transition
coexistence
& jump-like
transition
coexistence
(ΔE/kT*)(ΔT/T*)
~ 1
Transition: |ΔF1|= |-ΔS×ΔT| ~ kT*
ΔE-T*ΔS=0
Слайд 15 LANDAU: Helix-coil transition:
Melting:
NOT 1-s order phase transition
1-s order phase transitionHelix & coil: 1D objects Ice & water: 3D objects
N
N
n
n
ΔFhelix_n = Const + n×f ΔFICE_n = C×n2/3 + n×f
1D interface 3D interface
Mid-transition: f = 0
ΔShelix_n ~ ln(N) positions ΔSICE_n ~ ln(N)
N : very large; n ~ αN, α<<1 (e.g., α~0.001)
Const << ln(N) α2/3⋅N2/3 >> ln(N)
phases mix phases do not mix
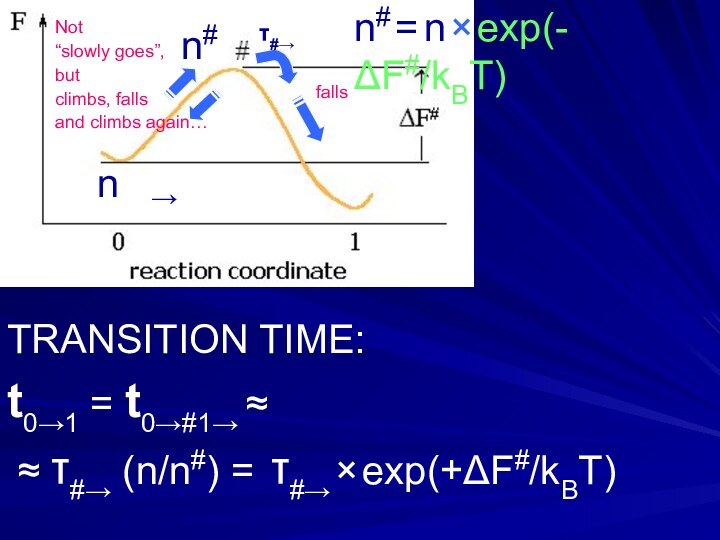
Слайд 18
n# = n × exp(-ΔF#/kBT)
n#
n
→
TRANSITION TIME:
t0→1 =
t0→#1→ ≈
≈ τ#→ (n/n#) =
τ#→ × exp(+ΔF#/kBT)Not
“slowly goes”,
but
climbs, falls
and climbs again…
falls
τ#→
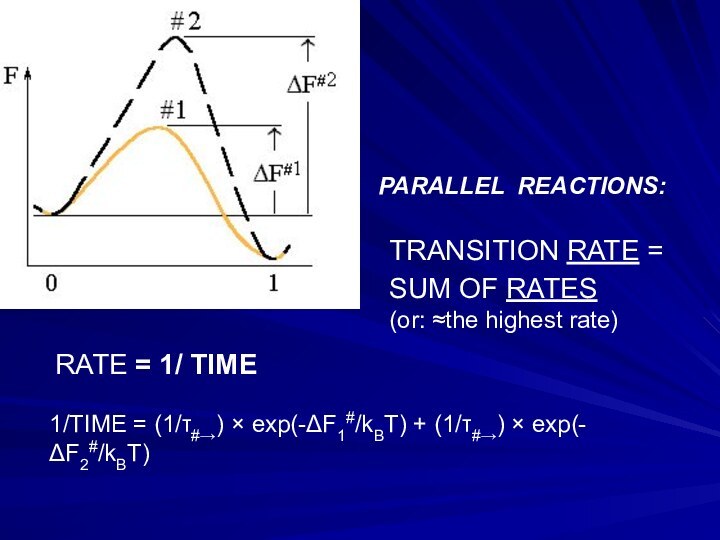
Слайд 21 TRANSITION RATE = SUM OF RATES
(or: ≈the highest rate)
1/TIME = (1/τ#→) × exp(-ΔF1#/kBT) + (1/τ#→) × exp(-ΔF2#/kBT)
PARALLEL REACTIONS:
RATE = 1/ TIME
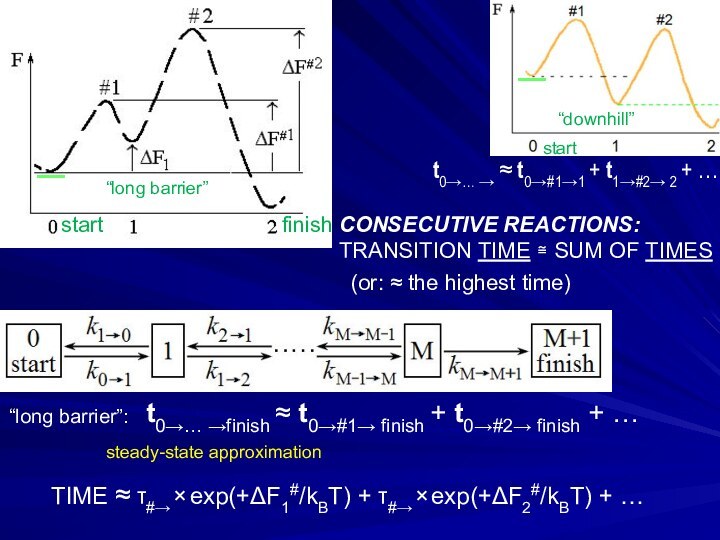
Слайд 22 t0→… →finish ≈ t0→#1→ finish + t0→#2→ finish
+ …
#
#
start
_
CONSECUTIVE REACTIONS:
TRANSITION TIME ≅ SUM OF TIMES
(or:
≈ the highest time)TIME ≈ τ#→ × exp(+ΔF1#/kBT) + τ#→ × exp(+ΔF2#/kBT) + …
steady-state approximation
t0→… → ≈ t0→#1→1 + t1→#2→ 2 + …
start
_
“long barrier”
“downhill”
“long barrier”:
finish
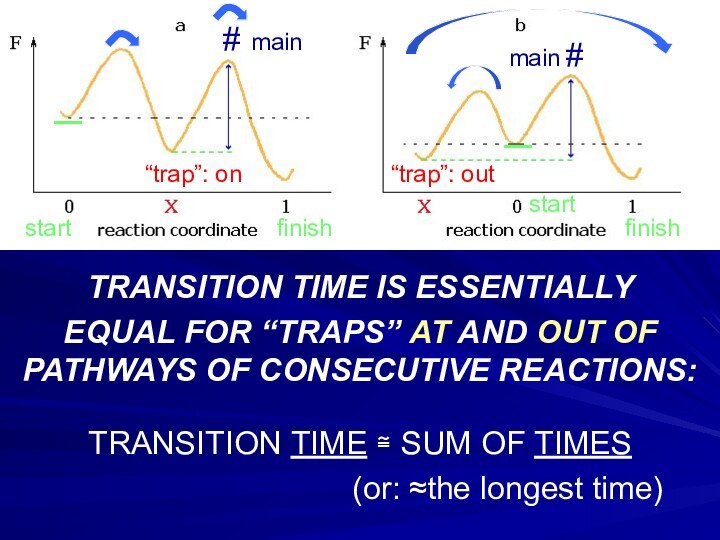
Слайд 23
_
_
TRANSITION TIME IS ESSENTIALLY
EQUAL FOR “TRAPS” AT
AND OUT OF PATHWAYS OF CONSECUTIVE REACTIONS:
TRANSITION TIME ≅
SUM OF TIMES(or: ≈the longest time)
# main
finish
finish
start
start
“trap”: on
“trap”: out
main #
Слайд 25 Mean kinetic energy of a particle:
~ kBT = Σj Pj(εj) ∙ εj
v2 = (vX2)+(vY2)+(vZ2) Maxwell :in 3D
James Clerk
(1831 –1879)
Слайд 26
Friction stops a molecule within picoseconds:
m(dv/dt) =
-(3πDη)v [Stokes law], or m(dv/dt) = -(kBT/Ddiff)v
[Einstein-Stokes]
D – diameter;
m ~ D3 ⋅ 1g/cm3 – mass;
η – viscosity
tkinet ≈ 10-13 sec × (D/nm)2
in water
Sir George Gabriel Stokes Albert Einstein
DIFFUSION: (1819-1903) (1879-1995)
During tkinet the molecule moves by Lkinet ~ v•tkinet
Then it restores its kinetic energy mv2/2 ~ kBT from thermal kicks of other molecules, and moves in another random side
CHARACTERISTIC DIFFUSION TIME: nanoseconds
Слайд 27
Friction stops a molecule within picoseconds:
tkinet
≈ 10-13 sec × (D/nm)2 in waterDIFFUSION:
During tkinet the molecule moves by Lkinet ~ v•tkinet
Then it restores its kinetic energy mv2/2 ≈ kBT from thermal kicks
of other molecules, and moves in another
random side
CHARACTERISTIC DIFFUSION
TIME: nanoseconds
The random walk allows the molecule
to diffuse at distance D (~ its diameter)
within ~(D/L kinet)2 steps, i.e., within
tdifft ≈ tkinet•(D/Lkinet)2 = D2/Ddiff
≈ 4•10-10 sec × (D/nm)3 in water
r1
→
Слайд 29
For “small part”: Pj(εj) = exp(-εj/kBT)/Z(T);
Z(T) = Σj exp(-εj/kBT)
Σj Pj(εj) = 1
E(T) = <ε> = Σj εj∙ Pj(εj)
if all εj = ε : #STATES = 1/P, i.e.: S(T) = kB∙ln(1/P)
S(T) = kB
F(T) = E(T) - TS(T) = -kBT ∙ ln[ Z(T)]
STATISTICAL MECHANICS
Слайд 30
Thermostat: Tth = dEth/dSth
“Small part”: Pj(εj,Tth) ~
exp(-εj/kBTth);
E(Tth) = Σj εj Pj(εj,Tth) S(Tth) = kBΣj ln[1/Pj(εj,Tth)]Pj(εj,Tth)
Tsmall_part = dE(Tth)/dS(Tth) = Tth
STATISTICAL
MECHANICS
Слайд 32
Separation of potential energy
in classic (non-quantum) mechanics:
P(ε) ~
exp(-ε/kBT) Classic: ε = εCOORD +
εKINεKIN = mv2/2 : does not depend on coordinates
Potential energy εCOORD: depends only on coordinates
P(ε) ~ exp(-εCOORD/kBT) • exp(-εKIN/kBT)
ZKIN(T) = ΣK exp(-εK/kBT): don’t depend on coord.
ZCOORD(T) = ΣCexp(-εC/kBT): depends on coord.
Z(T) = ZCOORD(T)•ZKIN(T) ⇒ F(T) = FCOORD(T)+FKIN(T)
========================================================================================================================
Elementary volume: Δ(mv)Δx = ħ ⇒ (Δx)3 =(ħ/|mv|)3
Слайд 33
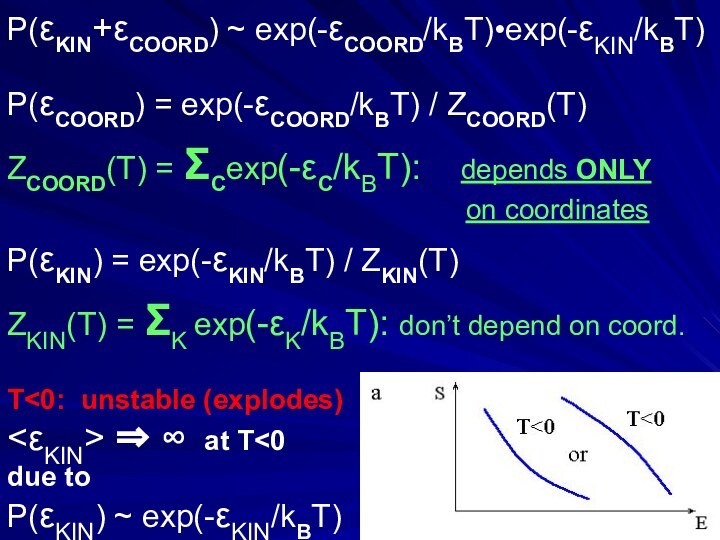
P(εKIN+εCOORD) ~ exp(-εCOORD/kBT)•exp(-εKIN/kBT)
P(εCOORD) = exp(-εCOORD/kBT) / ZCOORD(T)
ZCOORD(T) =
ΣCexp(-εC/kBT): depends ONLY
on coordinatesP(εKIN) = exp(-εKIN/kBT) / ZKIN(T)
ZKIN(T) = ΣK exp(-εK/kBT): don’t depend on coord.
T<0: unstable (explodes)
<εKIN> ⇒ ∞ at T<0
due to
P(εKIN) ~ exp(-εKIN/kBT)





























