- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему спирть
Содержание
- 2. ALCOHOLS (alcohols) - a class of organic
- 3. Classification of alcohols varied and depends on
- 4. Compounds in which one carbon atom has
- 5. 2. The type of carbon atom which
- 6. Fig. 2. THE STRUCTURE OF TERTIARY ALCOHOLSC)
- 7. 3. According to the structure of the
- 8. Nomenclature of alcoholsFor common alcohols having a
- 9. In the case where the structure of
- 10. Functional (HE) and substitute (CH3) group, and
- 11. Physical properties of alcoholsAlcohols are soluble in
- 12. Chemical properties of alcoholsAlcohols differ in various
- 13. Obtaining alcohols. Some of the reactions shown
- 14. Ethanol is formed and the so-called alcoholic
- 15. The use of alcohols.The ability of alcohols
- 16. Methanol CH3OH is used as a solvent
- 17. Ethanol SNO - source connection for receiving
- 18. Butane is used as solvent for fats
- 19. Finitely alcohol SN-CH2-CH2-OH has the smell of
- 20. Glycerin HOCH2-CH(OH)-CH2OH used to produce polyester glyptic
- 21. Скачать презентацию
- 22. Похожие презентации
ALCOHOLS (alcohols) - a class of organic compounds containing one or more groups-HE, with the hydroxyl group linked to an aliphatic carbon atom (compounds in which the carbon atom in the group WITH-IT is part of



















Слайд 3 Classification of alcohols varied and depends on the
sign of the structure taken as a basis
1. Depending
on the number of hydroxyl groups in the molecule, alcohols are divided into:
a) monatomic (consist of a single hydroxyl IT group), for example, methanol CH3OH, ethanol SON, propane SON
b) polyatomic (two or more hydroxyl groups), for example, ethylene glycol
HO-CH2-CH2-OH, glycerin HO-CH2-CH(Oh)-CH2-OH, pentaerythritol C(SNON)4.Слайд 4 Compounds in which one carbon atom has two
hydroxyl groups, in most cases, unstable and easily converted
into aldehydes, replay with water: RCH(OH)2 ® RCH=O + H2OAlcohols containing three groups HE has one carbon atom , do not exist.
Слайд 5 2. The type of carbon atom which is
connected with the group HE, alcohols are divided into: (a)
primary, which group is linked to a primary carbon atom. The primary is called the carbon atom (red) is associated with only one carbon atom. Examples of primary alcohols - ethanol CH3-CH2-OH, propane CH3-CH2-CH2-OH. b) secondary, which group is linked to a secondary carbon atom. The secondary carbon atom (highlighted in blue) is associated simultaneously with two carbon atoms, for example, secondary propel alcohol, secondary butane (Fig. 1).Fig. 1. THE STRUCTURE OF SECONDARY ALCOHOLS
Слайд 6
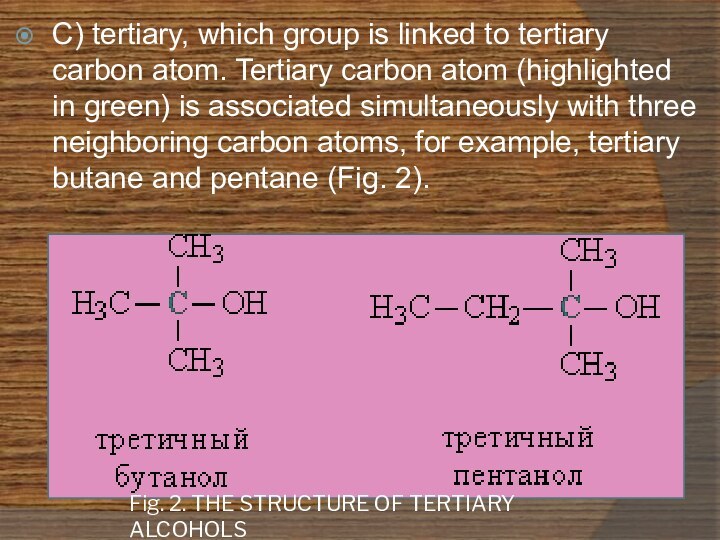
Fig. 2. THE STRUCTURE OF TERTIARY ALCOHOLS
C) tertiary,
which group is linked to tertiary carbon atom. Tertiary
carbon atom (highlighted in green) is associated simultaneously with three neighboring carbon atoms, for example, tertiary butane and pentane (Fig. 2).Слайд 7 3. According to the structure of the organic
groups connected HE is in the group, the alcohols
are divided into marginal (methanol, ethanol, propane), unsaturated, for example, ally alcohol CH2=CH-CH2 -, HE, aromatic (e.g., benzyl alcohol SNNAN) containing the group R is an aromatic group. Unsaturated alcohols, for whom HE is the group adjacent to the double bond, i.e. linked to the carbon atom participating simultaneously in the formation of double bond (e.g., vinyl alcohol CH2=CH-OH), a highly unstable and immediately are isomerizes (see ISOMERIZATION) to aldehydes or ketenes:CH2=CH–OH ® CH3–CH=O
Слайд 8
Nomenclature of alcohols
For common alcohols having a simple
structure, simplified nomenclature: the name of the organic group
is converted to an adjective (with the help of the suffix and the end of the "new" and add the word "alcohol":СН3ОН methyl alcohol
С2Н5ОН ethyl alcohol
(Н3С)2СНОН isopropyl alcohol
С4Н9ОН butyl alcohol
Слайд 9 In the case where the structure of the
organic group is more complex, use all organic chemistry
rules. Names, made in such rules, called systematic. In accordance with these rules, the hydrocarbon chain is numbered from the end nearer HE is group. Then use this numbering to indicate the position of different substituent's in the main chain, at the end of the title add the suffix "old" and a number, indicating the position of Oh groups (Fig. 4):Fig. 4. SYSTEMATIC NAMES OF ALCOHOLS
Слайд 10 Functional (HE) and substitute (CH3) group, and the
corresponding digital indices of selected distinct colors. Systematic names of
the simplest alcohols are by the same rules: methanol, ethanol, butane. For some spirits remained trivial (simplified) names, historically: property alcohol NCS-CH2 -, HE, glycerin HO-CH2-CH(Oh)-CH2-OH, pentaerythritol C(SNO)4, finitely alcohol SN-CH2-CH2-OH.
Слайд 11
Physical properties of alcohols
Alcohols are soluble in most
organic solvents, the first three of the simplest representative
- methanol, ethanol and propane, and tertiary butane (NS)SON - mixed with water in any ratio. When the number of atoms in the organic group is beginning to affect hydrophobic (water-repellent) effect, the solubility in water becomes limited, and in the case of R containing more than 9 carbon atoms, practically disappears. Due to the presence of Oh groups between molecules of alcohols hydrogen bonds occur. The result of all alcohols higher boiling point than the corresponding hydrocarbons, for example, Kip. ethanol +78° C, and T. Kip. ethane -88,63° C; b. p .. butane and butane respectively +117,4° C and -0.5° C.Fig. 5. HYDROGEN bonds IN ALCOHOLS (shown by a dotted line)
Слайд 12
Chemical properties of alcohols
Alcohols differ in various transformations.
Reactions of alcohols have some common patterns: reactivity of
primary Monohydric alcohols are higher than the secondary, in turn, secondary alcohols are chemically more active than tertiary. For dihydric alcohols, in the case when the Oh groups are located at adjacent carbon atoms, there is increased (in comparison with Monohydric alcohols) reactivity due to the mutual influence of these groups. For alcohols the possible reactions taking place with a gap as C-O and O-H - bonds. When interacting with mineral or organic acids alcohols to form esters are compounds containing the fragment R-O-A (a - acid residue). The formation of esters occurs by the interaction of alcohols with anhydrides and chlorides of carboxylic acids (Fig. 6).
Слайд 13
Obtaining alcohols.
Some of the reactions shown above
(Fig. 6,9,10) reversible and when conditions change can proceed
in the opposite direction, causing them to produce alcohol, for example by the hydrolysis of esters and kalogeropoulou (Fig.11A and B, respectively), as well as the hydration of alkenes - attach water (Fig.11B).Fig. 11. OBTAINING ALCOHOLS BY HYDROLYSIS AND HYDRATION OF ORGANIC COMPOUNDS
Reaction of alkenes hydrolysis (Fig. 11, scheme C) is the basis of industrial production of lower alcohols containing up to 4 atoms of C.





























