Слайд 2
What is Bioremediation??
Using subsurface microorganisms to transform hazardous
contaminants into relatively harmless byproducts, such as ethene and
water
Biodegrade
Mineralize
Biotransform
Techniques or types of bioremediation:
A component of Natural Attenuation
Enhanced Bioremediation
Bioaugmentation
Слайд 3
Bioremediation Background
Natural Attenuation is Not fast enough, Not
complete enough, Not frequently occurring enough to be broadly
used for some compounds, especially chlorinated solvents
The current trend is to stimulate/enhance a site’s indigenous subsurface microorganisms by the addition of nutrients and electron donor
In some cases, bioaugmentation is necessary when metabolic capabilities are not naturally present.
Слайд 4
Historical Perspective
~1900 Advent of biological processes to treat
organics derived from human or animal wastes (and the
sludges produced)
~1950 Approaches to extend wastewater treatment to industrial wastes
~1960 Investigations into the bioremediation of synthetic chemicals in wastewaters
~1970 Application in hydrocarbon contamination such as oil spills and petroleum in groundwater
~1980 Investigations of bioremediation applications for substituted organics
~1990 Natural Attenuation of ’70 and ’90, and the development of barrier approaches
~2000 High-rate in situ bioremediation; source zone reduction; bioaugmentation
Слайд 5
Soil and Subsurface Contaminants
Benzene and related fuel components
(BTEX)
Pyrene and other polynuclear aromatics
Chlorinated aromatics and solvents
Herbicides and
pesticides
Nitroaromatic explosives and plasticizers
Слайд 6
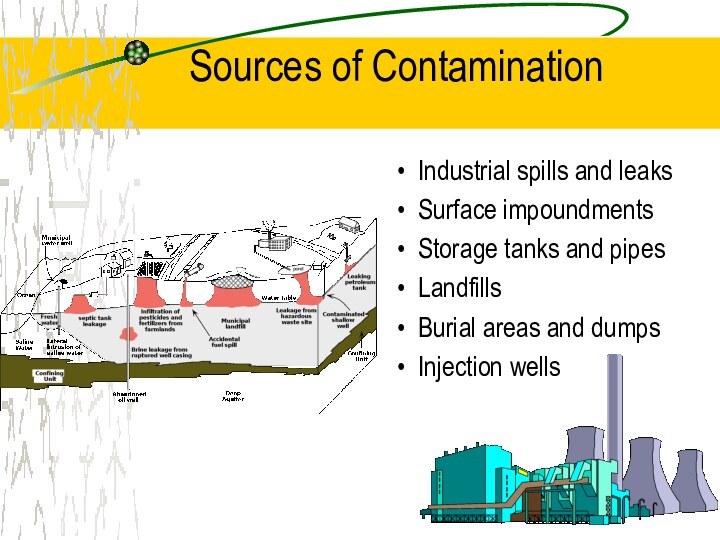
Sources of Contamination
Industrial spills and leaks
Surface impoundments
Storage tanks
and pipes
Landfills
Burial areas and dumps
Injection wells
Слайд 7
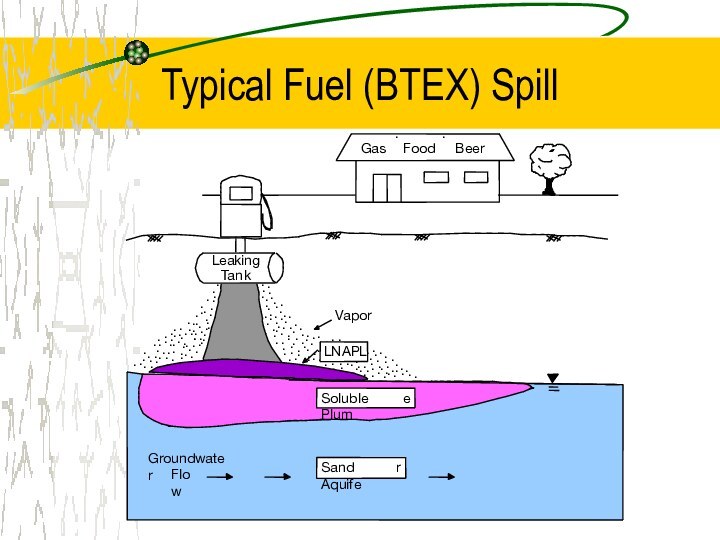
Current Water Issues Associated with Gasoline Use
Widespread contamination
Major
treat to drinking water resources
Components of fuels are known
carcinogens
Current fuel oxygenate, MTBE, very mobile and not very degradable
Ethanol is due to replace MTBE, but its behavior in the subsurface is not yet understood
Слайд 9
Chlorinated Background
Groundwater plumes of chlorinated solvents are widespread
due to their extensive use at industrial, DOD, and
dry cleaner sites.
Chlorinated compounds commonly exist as dense nonaqueous-phase liquids (DNAPLs) that act as long-term, continuing sources that slowly solubilize into groundwater.
Known carcinogenic and toxic effects
Not a primary substrate for any known bacteria
Слайд 11
DNAPL
Our Most Difficult Challenge
DNAPL source
Residual phase
Trapped on
lenses
Pools in low areas
Creates soluble plumes for years
Extremely hard
to remediate
Слайд 12
Treatment Techniques
Soil Extraction
Pump and Treat
Physical and/or reactive
barriers
Air and Hydrogen Sparging
Biological (microbes)
Chemical (surfactants)
Слайд 13
Why use Bioremediation?
No additional disposal costs
Low maintenance
Does not
create an eyesore
Capable of impacting source zones and thus,
decreasing site clean-up time
Слайд 14
Source Zone Treatment vs.
Plume Treatment
Слайд 15
Fundamentals of Biodegradation
All organics are biodegradable, BUT biodegradation
requires specific conditions
There is no Superbug
Contaminants must be
bioavailable
Biodegradation rate and extent is controlled by a “limiting factor”
Слайд 16
Biotic Transformations
Result of metabolic activity of microbes
Aerobic and
anaerobic biodegradation
Reduces aqueous concentrations of contaminant
Reduction of contaminant mass
Most
significant process resulting in reduction of contaminant mass in a system
Слайд 17
Bioremediation Processes
Conversion of contaminants to mineralized (e.g. CO2,
H2O, and salts) end-products via biological mechanisms
Biotransformation refers to
a biological process where the end-products are not minerals (e.g., transforming TCE to DCE)
Biodegradation involves the process of extracting energy from organic chemicals via oxidation of the organic chemicals
Слайд 18
How Microbes Use the Contaminant
Contaminants may serve as:
Primary
substrate
enough available to be the sole energy source
Secondary
substrate
provides energy, not available in high enough concentration
Cometabolic substrate
fortuitous transformation of a compound by a microbe relying on some other primary substrate
Слайд 19
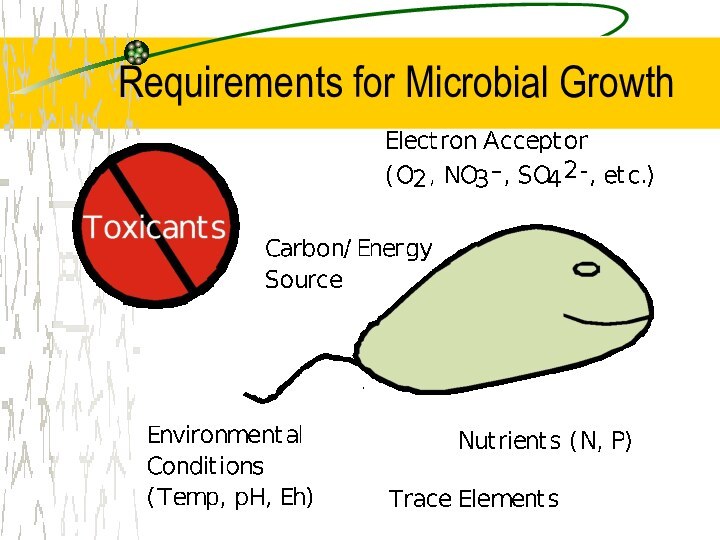
Requirements for Microbial Growth
Слайд 21
Aerobic v. Anaerobic
If oxygen is the terminal electron
acceptor, the process is called aerobic biodegradation
All other biological
degradation processes are classified as anaerobic biodegradation
In most cases, bacteria can only use one terminal electron acceptor
Facultative aerobes use oxygen, but can switch to nitrate in the absence of oxygen
Слайд 22
Aerobic
Oxidation
Cometabolism
Anaerobic
Denitrification
Manganese reduction
Iron
reduction
Sulfate reduction
Methanogenesis
Bacterial Metabolism
Слайд 23
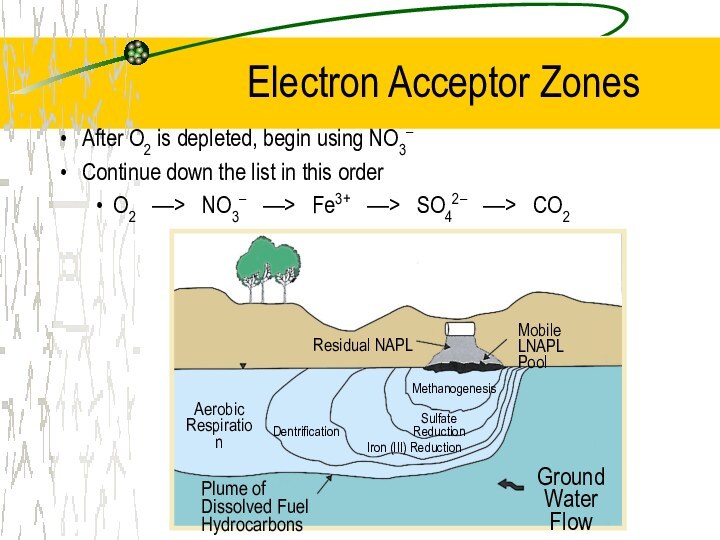
Electron Acceptor Zones
After O2 is depleted, begin using
NO3–
Continue down the list in this order
O2 ––>
NO3– ––> Fe3+ ––> SO42– ––> CO2
Слайд 25
Bioremediation Practice
Understand physical and chemical characteristics of the
contaminants of interest
Understand the possible catabolic pathways of metabolism
and the organisms that possess that capability
Understand the environmental conditions required to:
Promote growth of desirable organisms
Provide for the expression of needed organisms
Engineer the environmental conditions needed to establish favorable conditions and contact organisms and contaminants
Слайд 26
Oxygen is of Primary Importance
Most of the
time oxygen is the primary factor limiting in situ
biodegradation
In most cases if adequate oxygen can be supplied then biodegradation rates are adequate for remediation
Other limiting factors exist, but are usually secondary to oxygen
Degradation for Benzene: C6H6 + 7.5O2 ––> 6CO2 + 3H2O
Слайд 27
Two ways to introduce oxygen in situ
Dissolved
in water :
Actively pumped: H2 O2 , aerated
water
Passively: ORC ® , membrane, aeration
In gaseous form, usually air
Bioventing above the water table
Air sparging below the water table
Oxygen Supply is the Key to Aerobic
In Situ Bioremediation
Слайд 28
Dehalogenation
Stripping halogens (generally Chlorine) from an organic molecule
Generally
an anaerobic process, and is often referred to as
reductive dechlorination
R–Cl + 2e– + H+ ––> R–H + Cl–
Can occur via
Dehalorespiration (anaerobic)
Cometabolism (aerobic)
Слайд 29
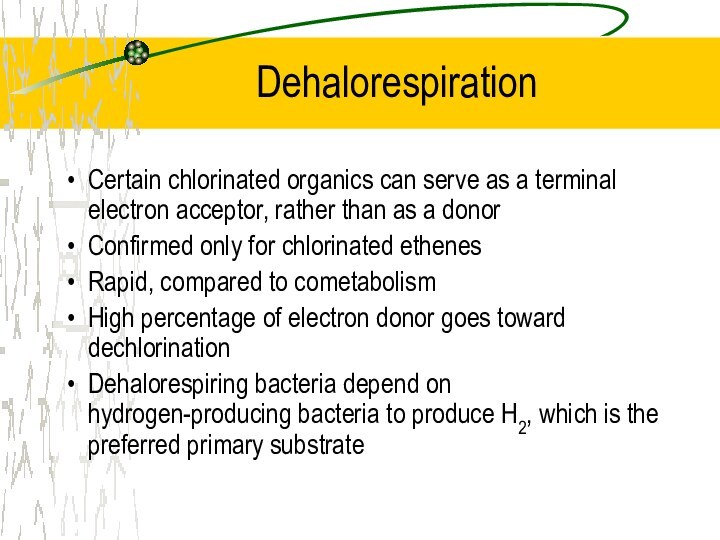
Dehalorespiration
Certain chlorinated organics can serve as a
terminal electron acceptor, rather than as a donor
Confirmed only
for chlorinated ethenes
Rapid, compared to cometabolism
High percentage of electron donor goes toward dechlorination
Dehalorespiring bacteria depend on hydrogen-producing bacteria to produce H2, which is the preferred primary substrate
Слайд 30
Reductive Dechlorination
An electron donor, such as hydrogen, and
an electron acceptor is needed to transfer from one
product to the next
Слайд 31
Added Danger
Dechlorination of PCE and TCE should be
encouraged, but monitored closely
The dechlorination products of PCE are
more hazardous than the parent compound
DCE is 50 times more hazardous than TCE
Vinyl Chloride is a known carcinogen
Слайд 32
Cometabolism
Fortuitous transformation of a compound by a microbe
relying on some other primary substrate
Generally a slow process
- Chlorinated solvents don’t provide much energy to the microbe
Most oxidation is of primary substrate, with only a few percent of the electron donor consumption going toward dechlorination of the contaminant
Not all chlorinated solvents susceptible to cometabolism (e.g., PCE and carbon tetrachloride)
Слайд 33
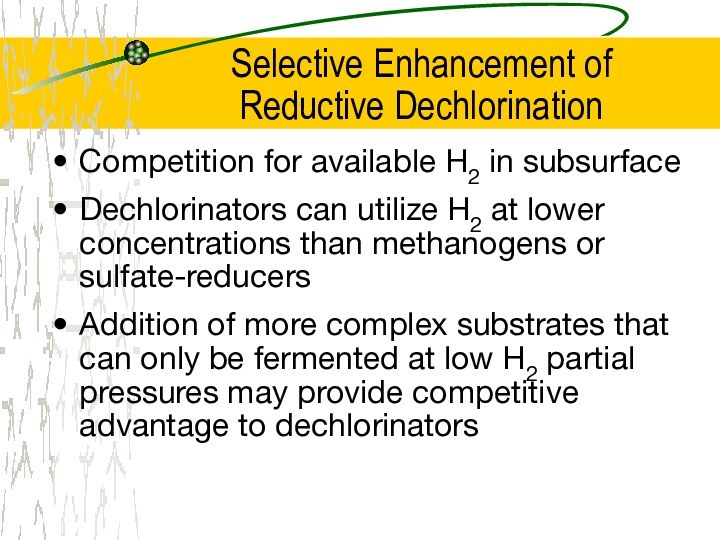
Selective Enhancement of
Reductive Dechlorination
Competition for available H2
in subsurface
Dechlorinators can utilize H2 at lower concentrations than
methanogens or sulfate-reducers
Addition of more complex substrates that can only be fermented at low H2 partial pressures may provide competitive advantage to dechlorinators
Слайд 34
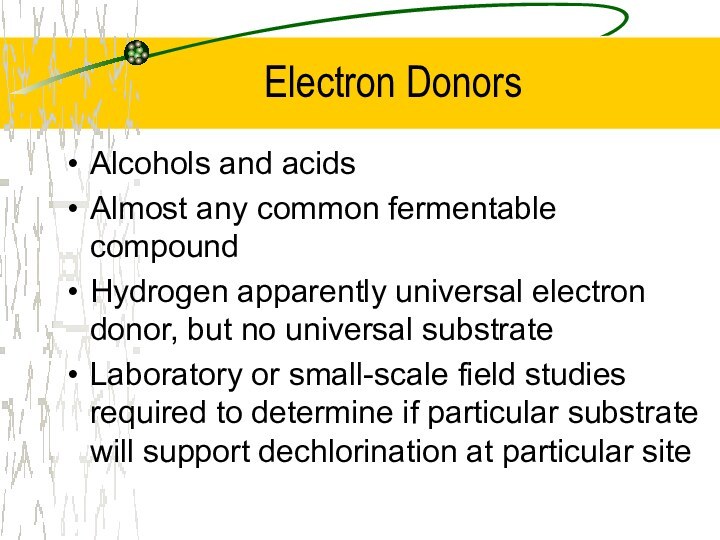
Electron Donors
Alcohols and acids
Almost any common fermentable compound
Hydrogen
apparently universal electron donor, but no universal substrate
Laboratory or
small-scale field studies required to determine if particular substrate will support dechlorination at particular site
Слайд 35
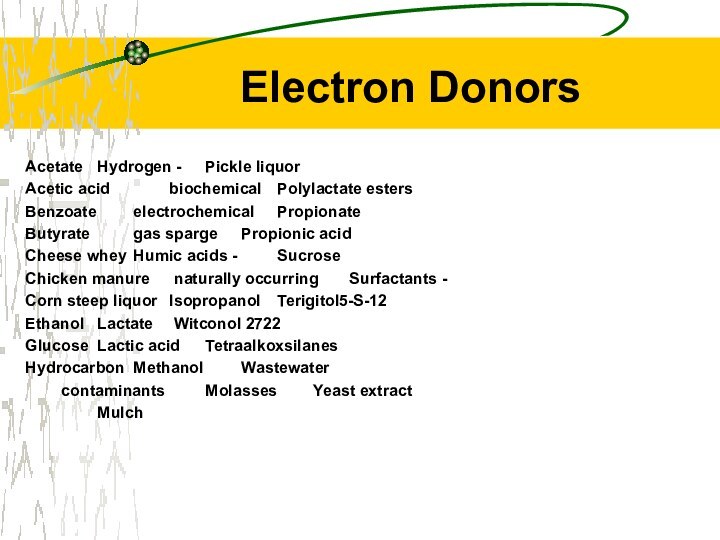
Electron Donors
Acetate Hydrogen - Pickle liquor
Acetic acid biochemical Polylactate esters
Benzoate electrochemical
Propionate
Butyrate gas sparge Propionic acid
Cheese whey Humic acids
- Sucrose
Chicken manure naturally occurring Surfactants -
Corn steep liquor Isopropanol Terigitol5-S-12
Ethanol Lactate Witconol 2722
Glucose Lactic acid Tetraalkoxsilanes
Hydrocarbon Methanol Wastewater
contaminants Molasses Yeast extract
Mulch
Слайд 36
Enhanced Bioattenuation
Petroleum Chlorinated
Technology Hydrocarbons Solvents
(e– acceptor) (e– donor)
Liquid Delivery Oxygen Benzoate
Nitrate
Lactate
Sulfate Molasses
Carbohydrates
Biosparge Air (oxygen) Ammonia
Hydrogen
Propane
Slow-release Oxygen Hydrogen
(ORC) (HRC)
Слайд 37
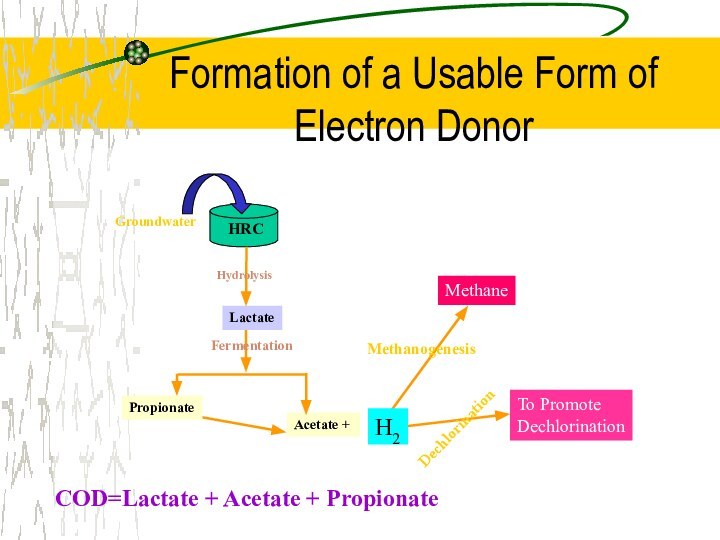
Formation of a Usable Form of Electron Donor
COD=Lactate + Acetate + Propionate