- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Heat flow and the first law of thermodynamics. Kind of thermodynamic process. Adiabatic processes
Содержание
- 2. Lecture 6 Heat flow and the first
- 3. HeatWhen the temperature of a thermal system
- 4. Mechanical equivalent of heatMechanical energy is not
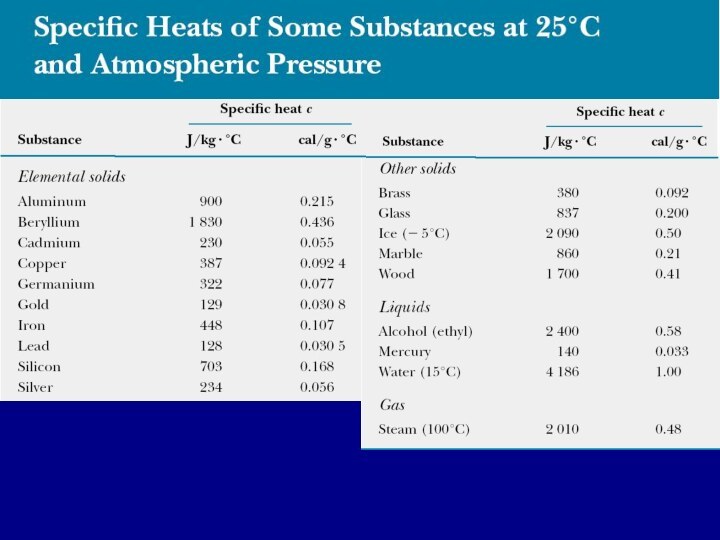
- 5. Specific heat capacityThe heat capacity C of
- 6. Energy transfer and specific heat capacityFrom this
- 8. Dependence of specific heat capacity on temperatureSpecific
- 9. Dependence of specific heat capacity on volume
- 10. Phase transitionIt can be that transfer of
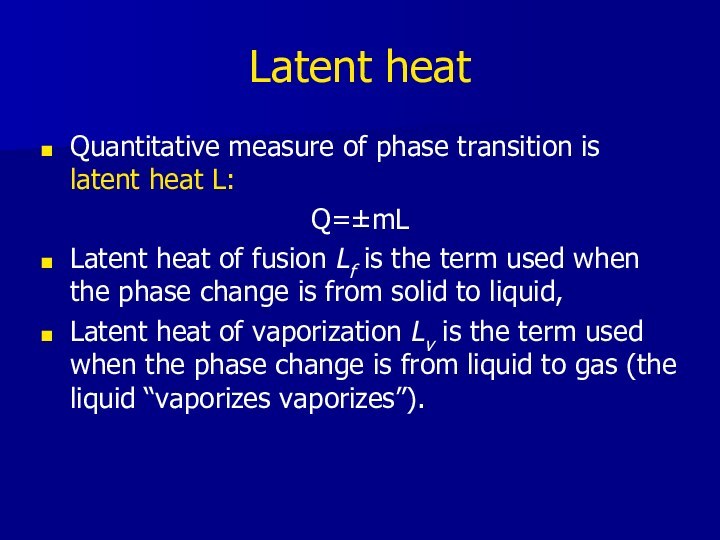
- 11. Latent heatQuantitative measure of phase transition is
- 13. State variables - Thermodynamic process - Thermal
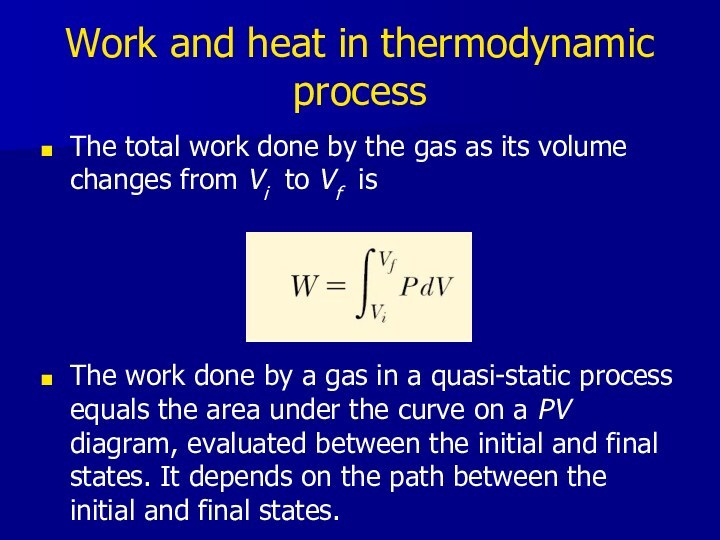
- 14. Work and heat in thermodynamic processThe total
- 15. Work depends on the path:(a): Wa= Pi(Vf-Vi)(b):
- 16. Two ways of energy transfer There exist two
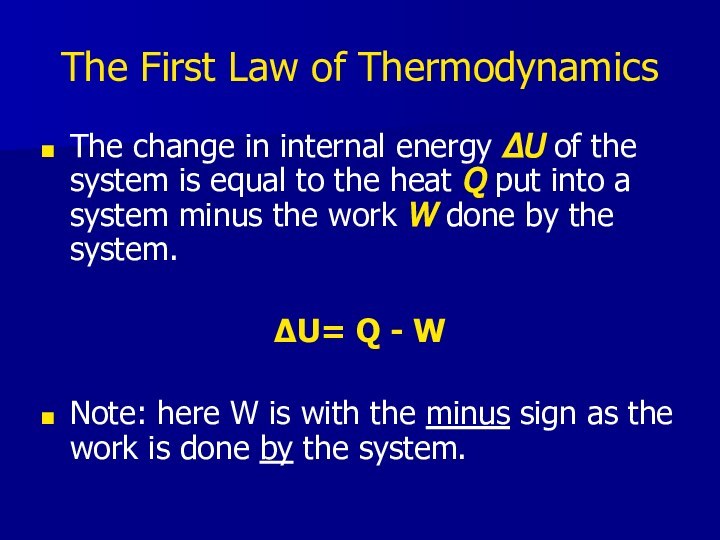
- 17. The First Law of ThermodynamicsThe change in
- 18. The first law of thermodynamics is a
- 19. Ideal Gas ProcessesHere W is work done
- 20. Adiabatic (no heat flow, Q=0):ΔW = -ΔU The
- 21. Polytropic processesPVγ = const, γ=const.Isobaric γ=0Isotermic γ=1Adiabatic γ= CP/CVIsochoric γ=∞
- 22. Cyclic ProcessesIf a nonisolated system is performing
- 23. Скачать презентацию
- 24. Похожие презентации
Lecture 6 Heat flow and the first law of thermodynamics. Kind of thermodynamic process. Adiabatic processes.















Слайд 3
Heat
When the temperature of a thermal system in
contact with a neighboring system changes, we say that
there has been a heat flow into or out of the system.An energy unit related to thermal processes is the calorie (cal), which is defined as the amount of energy transfer necessary to raise the temperature of 1 gram of water by 1 degree (from 14.5°C to 15.5°C).
Слайд 4
Mechanical equivalent of heat
Mechanical energy is not conserserved
in the presence of nonconservative forces. It transforms into
internal energy. For example, friction produces heating1 cal = 4.186 J
Слайд 5
Specific heat capacity
The heat capacity C of a
particular sample of a substance is defined as the
amount of energy needed to raise the temperature of that sample by 1 °C.C=Q/ΔΤ
The specific heat capacity c of a substance is the heat capacity per unit mass.
c=C/m=Q/(mΔΤ)
Specific heat is essentially a measure of how thermally insensitive a substance is to the addition of energy. The greater a material’s specific heat, the more energy must be added to a given mass of the material to cause a particular temperature change.
Слайд 6
Energy transfer and specific heat capacity
From this definition,
we can relate the energy Q transferred between a
sample of mass m and specific heat capacity c of a material and its surroundings to a temperature change ΔT asQ=mc ΔT
Слайд 8
Dependence of specific heat capacity on temperature
Specific heat
varies with temperature. For example, the specific heat of
water varies by only about 1% from 0 c °C to 100 °C at atmospheric pressure. Usually such variations are negligible.Слайд 9 Dependence of specific heat capacity on volume and
pressure
Measured values of specific heats are found to depend
on the conditions of the experiment. In general, measurements made in a constant pressure process are different from those made in a constant volume process. For solids and liquids, the difference between the two values is usually no greater than a few percent and is often neglected.
Слайд 10
Phase transition
It can be that transfer of energy
does not result in a change in emperature. This
is the case when the physical characteristics of the substance change from one form to another; such a change is called a phase change. Two common phase changes:melting: from solid to liquid
boiling: from liquid to gas
change in the crystalline structure of a solid
All such phase changes involve a change in internal energy but no change in temperature.
The increase in internal energy in boiling, for example, is represented by the breaking of bonds between molecules in the liquid state; this bond breaking allows the molecules to move farther apart in the gaseous state, with a corresponding increase in intermolecular potential energy.
Слайд 11
Latent heat
Quantitative measure of phase transition is latent
heat L:
Q=±mL
Latent heat of fusion Lf is the term
used when the phase change is from solid to liquid, Latent heat of vaporization Lv is the term used when the phase change is from liquid to gas (the liquid “vaporizes vaporizes”).
Слайд 13 State variables - Thermodynamic process - Thermal equilibrium
We describe the state of a system using such
variables as pressure, volume, temperature, and internal energy. These quantities are called state variables. Macroscopic state of a system can be specified only if the system is in thermal equilibrium. When we regard a thermodynamic process we imply that all its state variables change quasi-statically, that is, slowly enough to allow the system to remain essentially in thermal equilibrium at all times.
Слайд 14
Work and heat in thermodynamic process
The total work
done by the gas as its volume changes from
Vi to Vf isThe work done by a gas in a quasi-static process equals the area under the curve on a PV diagram, evaluated between the initial and final states. It depends on the path between the initial and final states.
Слайд 15
Work depends on the path:
(a): Wa= Pi(Vf-Vi)
(b): Wb=
Pf(Vf-Vi)
1) Wa< Wb as Pf < Pi
2) Wa
Wb as the coloured area in (b) case is large then the area in (a) case
Слайд 16
Two ways of energy transfer
There exist two ways
in which energy can be transferred between a system
and its surroundings:One way is work done by the system, which requires that there be a macroscopic displacement of the point of application of a force.
The other is heat, which occurs on a molecular level whenever a temperature difference exists across the boundary of the system.
Both mechanisms result in a change in the internal energy of the system and therefore usually result in measurable changes in the macroscopic variables of the system, such as the pressure, temperature, and volume of a gas.
Слайд 17
The First Law of Thermodynamics
The change in internal
energy ΔU of the system is equal to the
heat Q put into a system minus the work W done by the system.ΔU= Q - W
Note: here W is with the minus sign as the work is done by the system.
Слайд 18 The first law of thermodynamics is a special
case of the law of conservation of energy that
encompasses changes in internal energy and energy transfer by heat and work. It provides a connection between the microscopic and macroscopic approaches.
Слайд 19
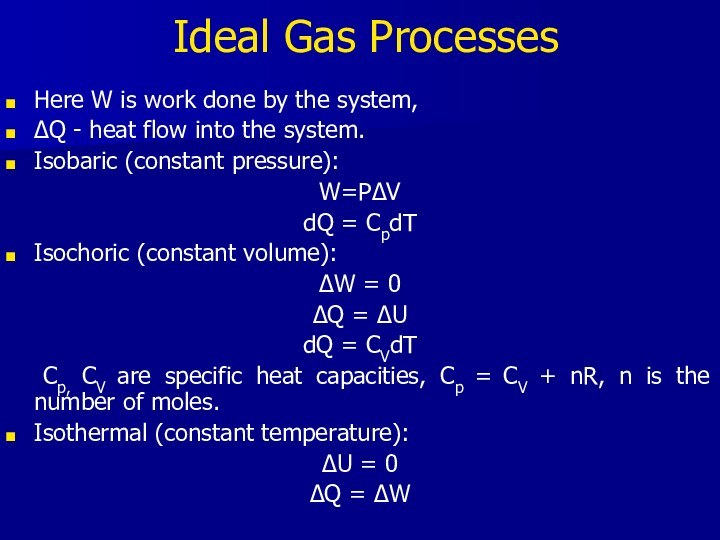
Ideal Gas Processes
Here W is work done by
the system,
ΔQ - heat flow into the system.
Isobaric
(constant pressure):W=PΔV
dQ = CpdT
Isochoric (constant volume):
ΔW = 0
ΔQ = ΔU
dQ = CVdT
Cp, CV are specific heat capacities, Cp = CV + nR, n is the number of moles.
Isothermal (constant temperature):
ΔU = 0
ΔQ = ΔW
Слайд 20
Adiabatic (no heat flow, Q=0):
ΔW = -ΔU
The curve
of adiabatic process is described by formula:
PVγ = const
TVγ−1
= constγ=CP/CV
Слайд 21
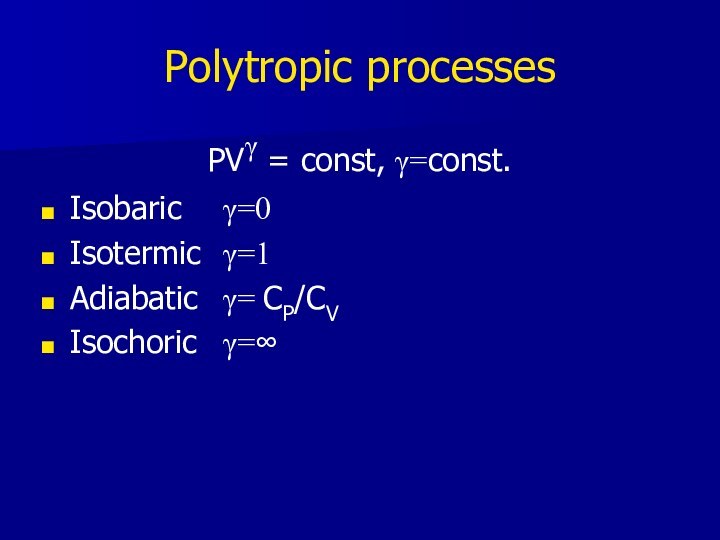
Polytropic processes
PVγ = const, γ=const.
Isobaric γ=0
Isotermic γ=1
Adiabatic γ= CP/CV
Isochoric γ=∞
Слайд 22
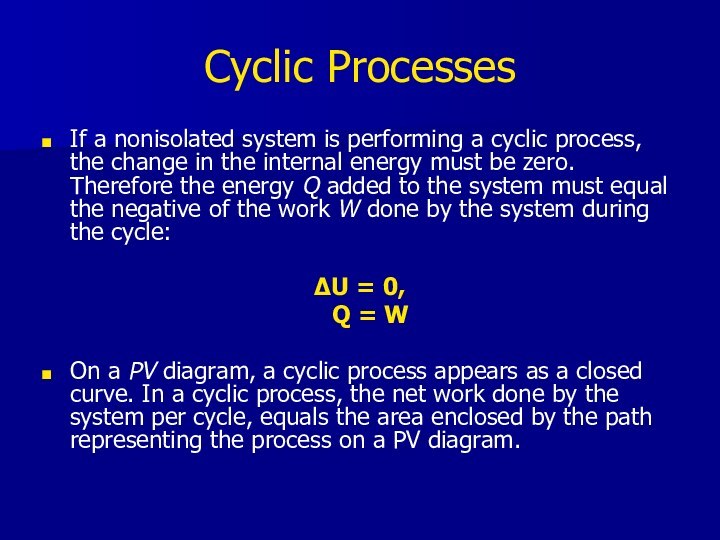
Cyclic Processes
If a nonisolated system is performing a
cyclic process, the change in the internal energy must
be zero. Therefore the energy Q added to the system must equal the negative of the work W done by the system during the cycle:ΔU = 0,
Q = W
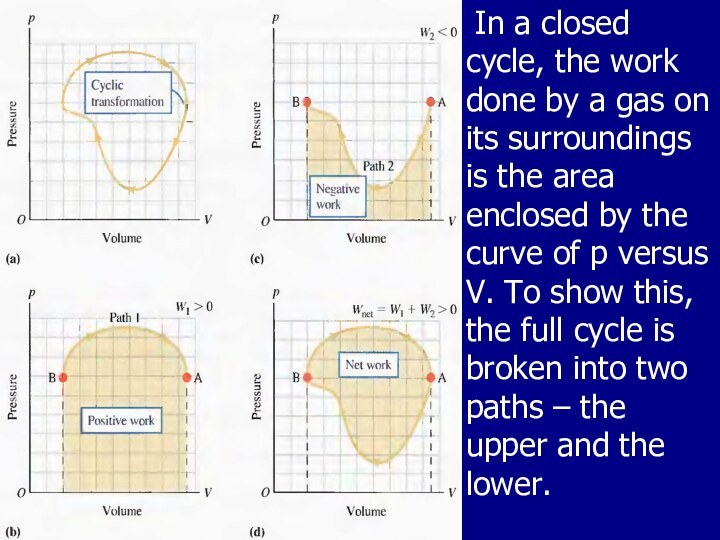
On a PV diagram, a cyclic process appears as a closed curve. In a cyclic process, the net work done by the system per cycle, equals the area enclosed by the path representing the process on a PV diagram.





























