- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Radiation
Содержание
- 2. Definition of Radiation“Radiation is an energy in
- 3. Forces: There are many interactions among nuclei.
- 4. Radioactivity: Elements & AtomsAtoms are composed of smaller particles referred to as:ProtonsNeutronsElectrons
- 5. Basic Model of a Neutral Atom.Electrons (-)
- 7. RadioactivityIf a nucleus is unstable for any
- 8. Ionization Ionizing radiation is produced by unstable
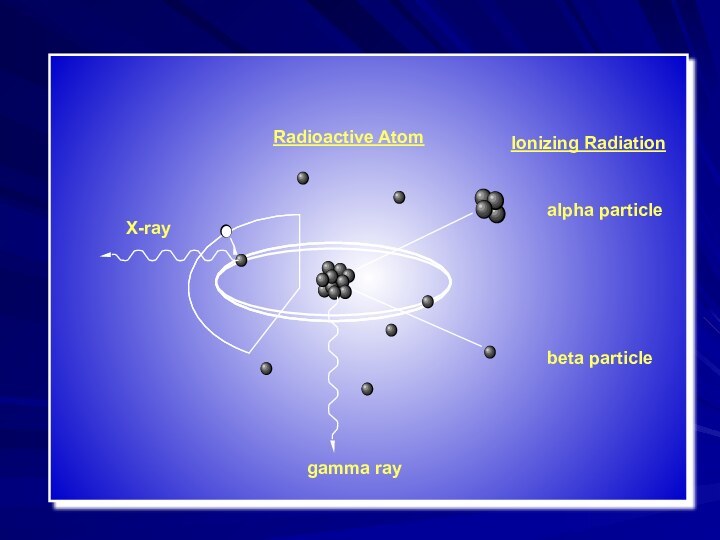
- 13. Types or Products of Ionizing Radiationβαγ or X-ray neutron
- 14. Radioactive AtomX-raygamma ray
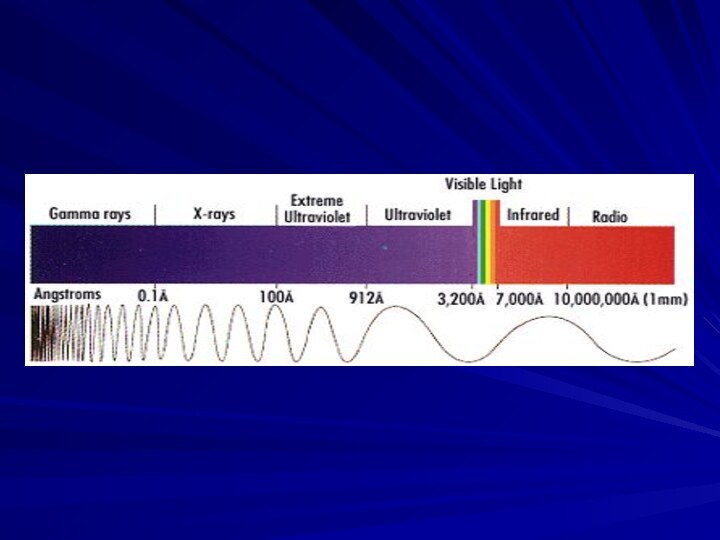
- 15. The electro-magnetic waves vary in their length and frequency along a very wide spectrum.
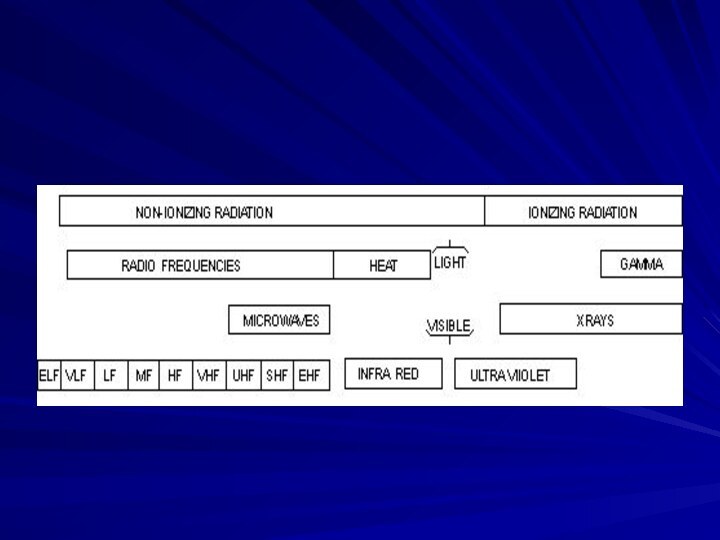
- 19. Types of RadiationRadiation is classified into:Ionizing radiationNon-ionizing radiation
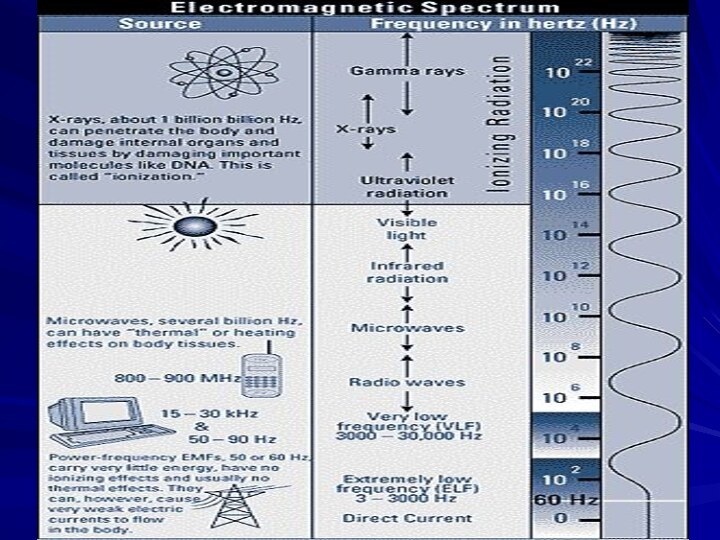
- 20. Ionizing Versus Non-ionizing RadiationIonizing RadiationHigher energy electromagnetic
- 21. Ionizing RadiationDefinition: “ It is a
- 22. Another DefinitionIonizing radiationA radiation
- 23. Primary Types of Ionizing RadiationAlpha particlesBeta particlesGamma rays (or photons)X-Rays (or photons)Neutrons
- 24. Alpha Particles: 2 neutrons and 2 protonsThey
- 25. Alpha Particles (or Alpha Radiation): Helium nucleus
- 26. Beta ParticlesBeta Particles: Electrons or positrons having
- 27. Beta Particles: High speed electron ejected from
- 29. Gamma RaysGamma Rays (or photons): Result when
- 30. X-RaysX-Rays: Occur whenever an inner shell orbital
- 31. X- and Gamma Rays: X-rays are photons
- 32. NeutronsNeutrons: Have the same mass as protons but are uncharged
- 35. QUANTIFICATION OF RADIATIONA. Quantifying Radioactive Decay B. Quantifying Exposure and Dose
- 36. A. Quantifying Radioactive DecayMeasurement of Activity in
- 37. B. Quantifying Exposure and DoseExposure: Roentgen 1
- 38. Half Life Calculation
- 39. Ionizing Radiation at the Cellular LevelCauses breaks
- 40. Exposure LimitsOSHA Limits: Whole body limit =
- 41. External/Internal Exposure Limits for Occupationally Exposed IndividualsAnnual Dose Limits*Effective dose equivalent
- 43. Community Emergency RadiationHazardous Waste Sites: Radiation above
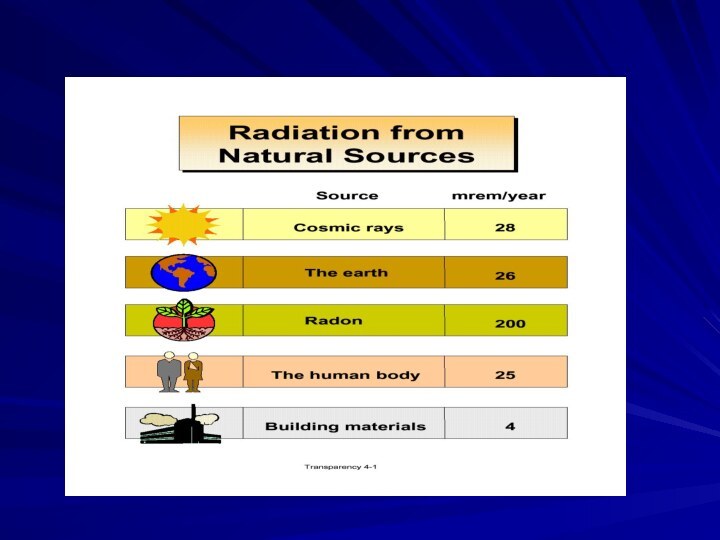
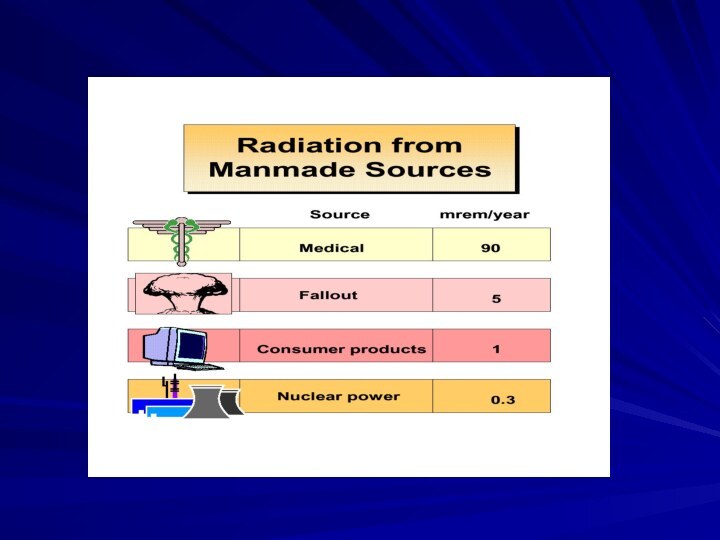
- 44. Your Annual Exposure
- 45. HEALTH EFFECTS Generalizations: Biological effects are due
- 46. ACUTE DOSE(RAD) EFFECT
- 47. Delayed Somatic Effects: Delayed effects to exposed
- 48. Critical Organs: Organs generally most susceptible to
- 49. Non-ionizing RadiationDefinition:“ They are electromagnetic waves incapable
- 50. All earth surface system components emit radiation---the
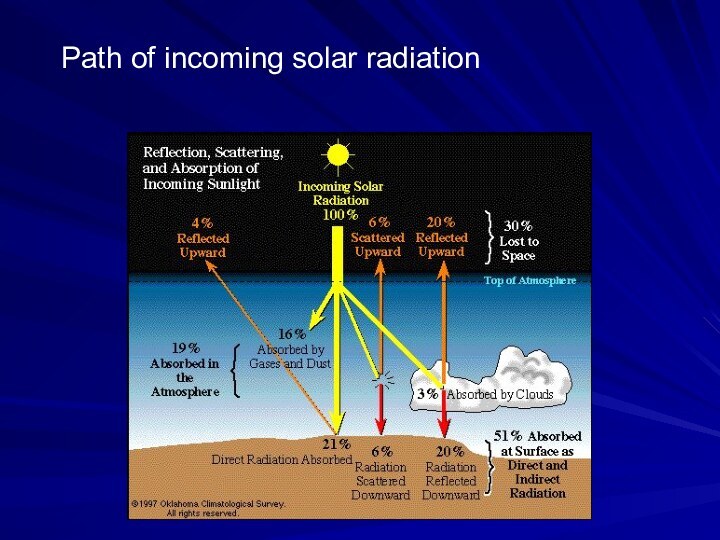
- 52. Path of incoming solar radiation
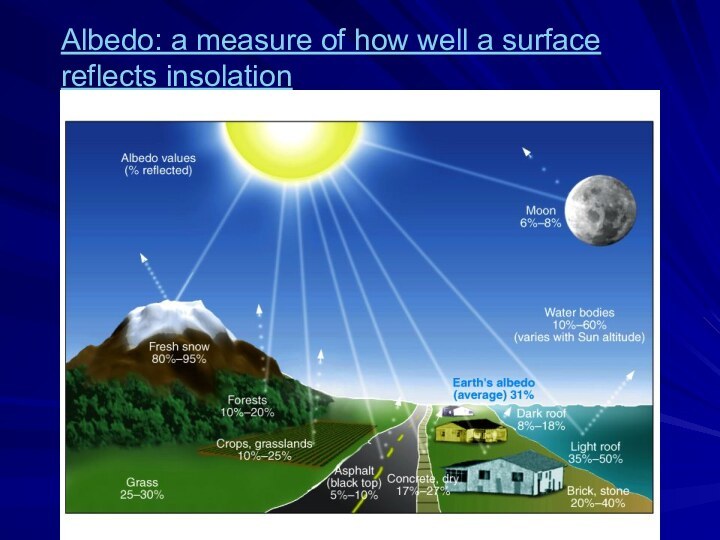
- 53. Albedo: a measure of how well a surface reflects insolation
- 54. Examples on Non-ionizing Radiation SourcesVisible lightMicrowavesRadiosVideo Display TerminalsPower linesRadiofrequency Diathermy (Physical Therapy)Lasers
- 55. Other Manmade Sources of Non-Ionizing Radiation
- 58. Effects Radiofrequency Ranges (10 kHz to 300
- 59. RADIATION CONTROLSA. Basic Control Methods for External Radiation Decrease Time Increase Distance Increase Shielding
- 60. Time: Minimize time of exposure to minimize
- 62. B. Monitoring Personal Dosimeters: Normally they do
- 66. Direct Reading Survey Meters and Counters: Useful
- 68. Continuous Monitors: Continuous direct reading ionization detectors
- 69. Elements of Radiation Protection Program Monitoring of
- 70. Скачать презентацию
- 71. Похожие презентации
Definition of Radiation“Radiation is an energy in the form of electro-magnetic waves or particulate matter, traveling in the air.”














































Слайд 3 Forces: There are many interactions among nuclei. It
turns out that there are forces other than the
electromagnetic force and the gravitational force which govern the interactions among nuclei.Einstein in 1905m showed 2 more laws: energy/mass, and binding energy
Слайд 4
Radioactivity: Elements & Atoms
Atoms are composed of smaller
particles referred to as:
Protons
Neutrons
Electrons
Слайд 5
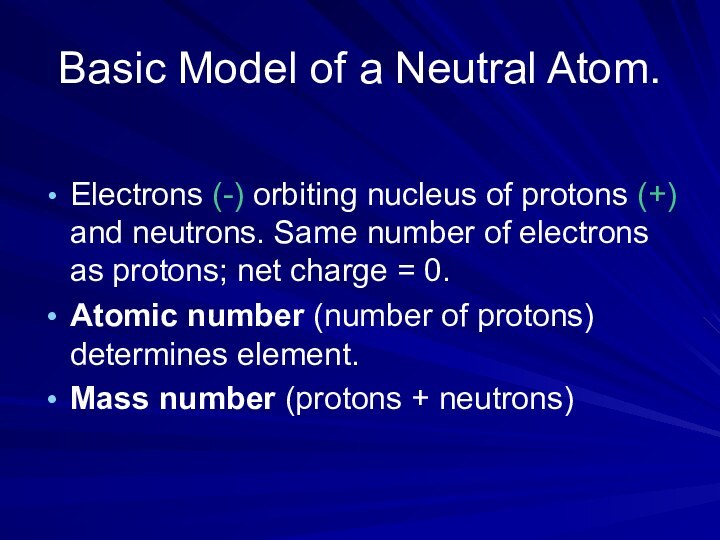
Basic Model of a Neutral Atom.
Electrons (-) orbiting
nucleus of protons (+) and neutrons. Same number of
electrons as protons; net charge = 0.Atomic number (number of protons) determines element.
Mass number (protons + neutrons)
Слайд 7
Radioactivity
If a nucleus is unstable for any reason,
it will emit and absorb particles. There are many
types of radiation and they are all pertinent to everyday life and health as well as nuclear physical applications.
Слайд 8
Ionization
Ionizing radiation is produced by unstable atoms.
Unstable atoms differ from stable atoms because they have
an excess of energy or mass or both.Unstable atoms are said to be radioactive. In order to reach stability, these atoms give off, or emit, the excess energy or mass. These emissions are called radiation.
Слайд 20
Ionizing Versus Non-ionizing Radiation
Ionizing Radiation
Higher energy electromagnetic waves
(gamma) or heavy particles (beta and alpha).
High enough energy
to pull electron from orbit.Non-ionizing Radiation
Lower energy electromagnetic waves.
Not enough energy to pull electron from orbit, but can excite the electron.
Слайд 21
Ionizing Radiation
Definition:
“ It is a
type of radiation that is able to disrupt atoms
and molecules on which they pass through, giving rise to ions and free radicals”.
Слайд 22
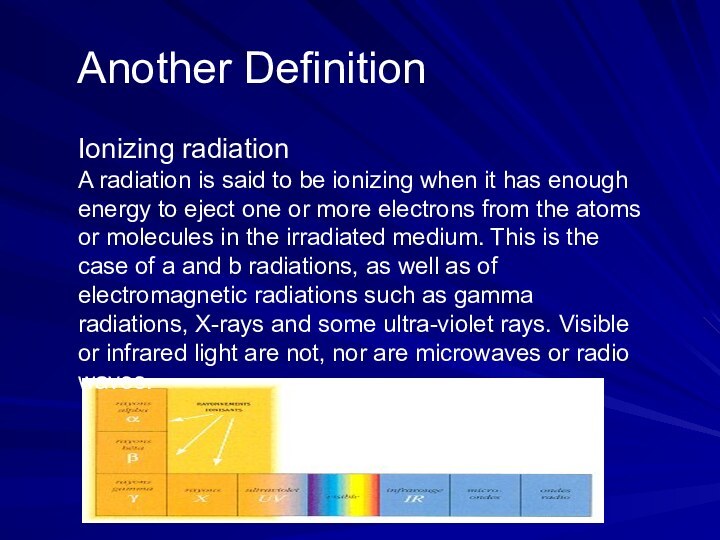
Another Definition
Ionizing radiation
A radiation is
said to be ionizing when it has enough energy
to eject one or more electrons from the atoms or molecules in the irradiated medium. This is the case of a and b radiations, as well as of electromagnetic radiations such as gamma radiations, X-rays and some ultra-violet rays. Visible or infrared light are not, nor are microwaves or radio waves.
Слайд 23
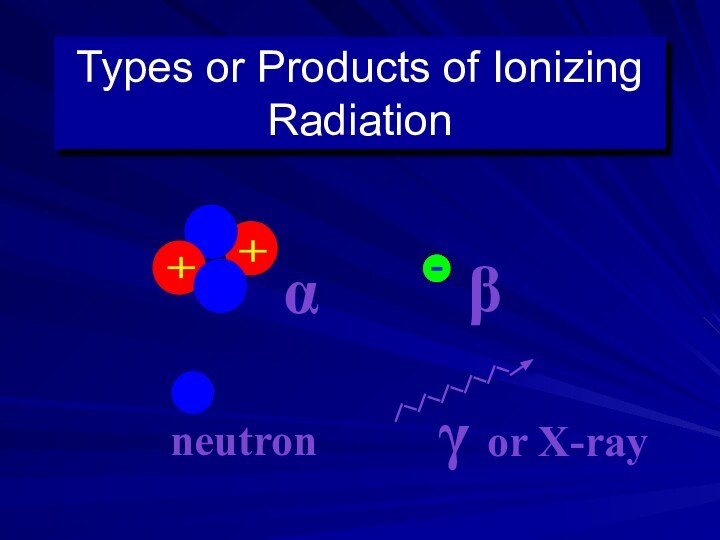
Primary Types of Ionizing Radiation
Alpha particles
Beta particles
Gamma rays
(or photons)
X-Rays (or photons)
Neutrons
Слайд 24
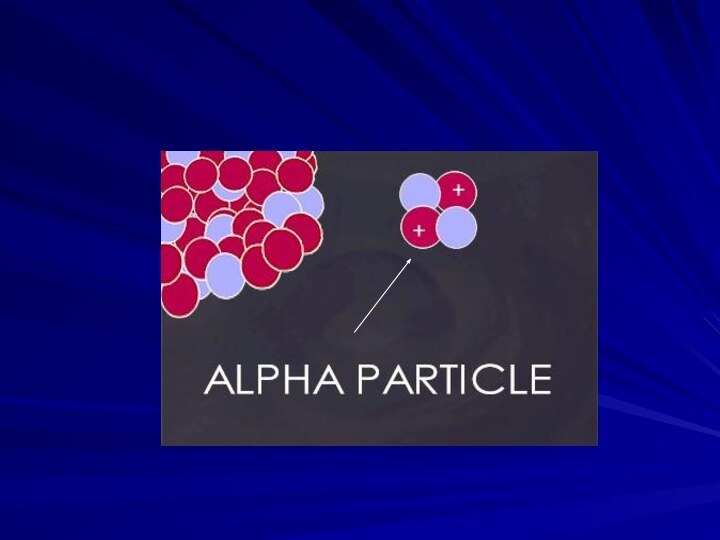
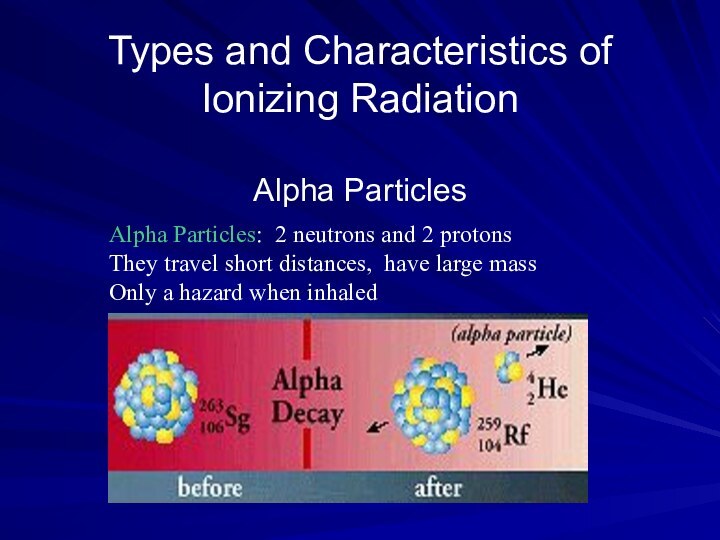
Alpha Particles: 2 neutrons and 2 protons
They travel
short distances, have large mass
Only a hazard when inhaled
Types
and Characteristics of Ionizing Radiation
Alpha Particles
Слайд 25
Alpha Particles (or Alpha Radiation): Helium nucleus (2
neutrons and 2 protons); +2 charge; heavy (4 AMU).
Typical Energy = 4-8 MeV; Limited range (<10cm in air; 60µm in tissue); High LET (QF=20) causing heavy damage (4K-9K ion pairs/µm in tissue). Easily shielded (e.g., paper, skin) so an internal radiation hazard. Eventually lose too much energy to ionize; become He.
Слайд 26
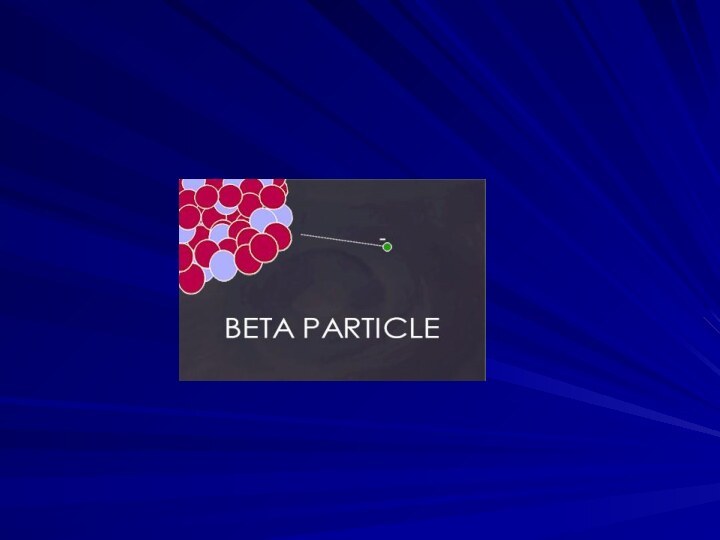
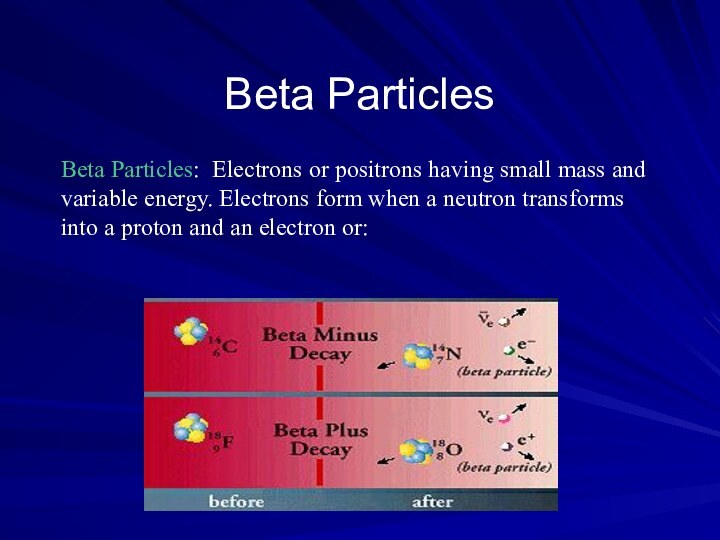
Beta Particles
Beta Particles: Electrons or positrons having small
mass and variable energy. Electrons form when a neutron
transforms into a proton and an electron or:
Слайд 27
Beta Particles: High speed electron ejected from nucleus;
-1 charge, light 0.00055 AMU; Typical Energy = several
KeV to 5 MeV; Range approx. 12'/MeV in air, a few mm in tissue; Low LET (QF=1) causing light damage (6-8 ion pairs/µm in tissue). Primarily an internal hazard, but high beta can be an external hazard to skin. In addition, the high speed electrons may lose energy in the form of X-rays when they quickly decelerate upon striking a heavy material. This is called Bremsstralung (or Breaking) Radiation. Aluminum and other light (<14) materials are used for shielding.
Слайд 29
Gamma Rays
Gamma Rays (or photons): Result when the
nucleus releases energy, usually after an alpha, beta or
positron transition
Слайд 30
X-Rays
X-Rays: Occur whenever an inner shell orbital electron
is removed and rearrangement of the atomic electrons results
with the release of the elements characteristic X-Ray energy
Слайд 31
X- and Gamma Rays: X-rays are photons (Electromagnetic
radiations) emitted from electron orbits. Gamma rays are photons
emitted from the nucleus, often as part of radioactive decay. Gamma rays typically have higher energy (Mev's) than X-rays (KeV's), but both are unlimited.
Слайд 35
QUANTIFICATION OF RADIATION
A. Quantifying Radioactive Decay
B. Quantifying
Exposure and Dose
Слайд 36
A. Quantifying Radioactive Decay
Measurement of Activity in disintegrations
per second (dps);
1 Becquerel (Bq) = 1 dps;
1 Curie (Ci) = 3.7 x 1010 dps;
Activity of substances are expressed as activity per weight or volume (e.g., Bq/gm or Ci/l).
Слайд 37
B. Quantifying Exposure and Dose
Exposure: Roentgen 1 Roentgen
(R) = amount of X or gamma radiation that
produces ionization resulting in 1 electrostatic unit of charge in 1 cm3 of dry air. Instruments often measure exposure rate in mR/hr.Absorbed Dose: rad (Roentgen absorbed dose) = absorption of 100 ergs of energy from any radiation in 1 gram of any material; 1 Gray (Gy) = 100 rads = 1 Joule/kg; Exposure to 1 Roentgen approximates 0.9 rad in air.
Biologically Equivalent Dose: Rem (Roentgen equivalent man) = dose in rads x QF, where QF = quality factor. 1 Sievert (Sv) = 100 rems.
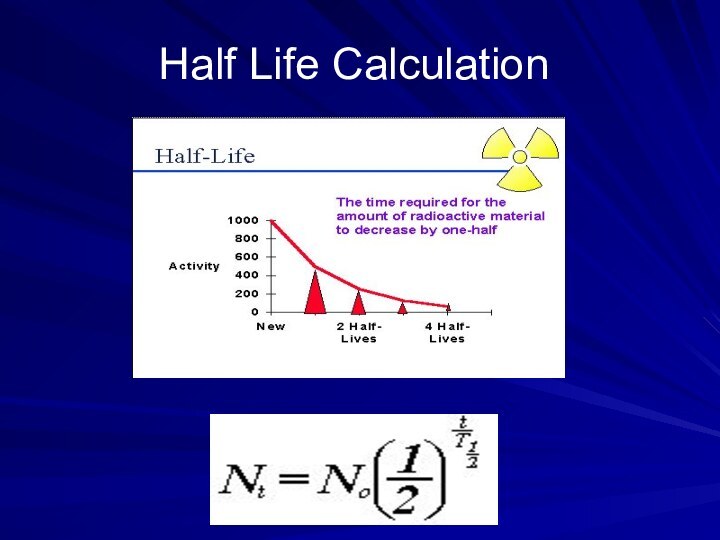
Слайд 39
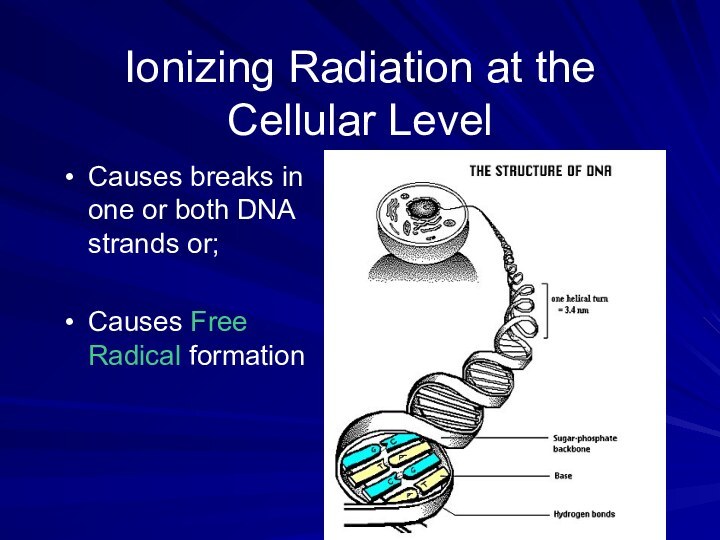
Ionizing Radiation at the Cellular Level
Causes breaks in
one or both DNA strands or;
Causes Free Radical formation
Слайд 40
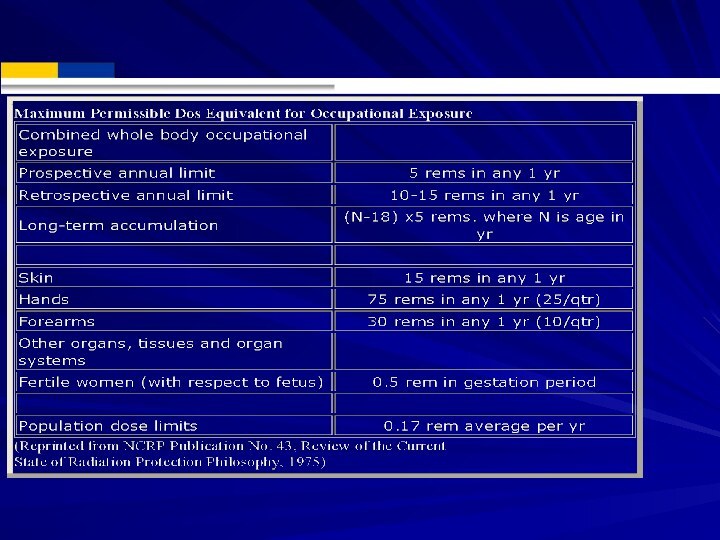
Exposure Limits
OSHA Limits: Whole body limit = 1.25
rem/qtr or 5 rem (50 mSv) per year.
Hands and
feet limit = 18.75 rem/qtr. Skin of whole body limit = 7.5 rem/qtr.
Total life accumulation = 5 x (N-18) rem where N = age. Can have 3 rem/qtr if total life accumulation not exceeded.
Note: New recommendations reduce the 5 rem to 2 rem.
Слайд 41
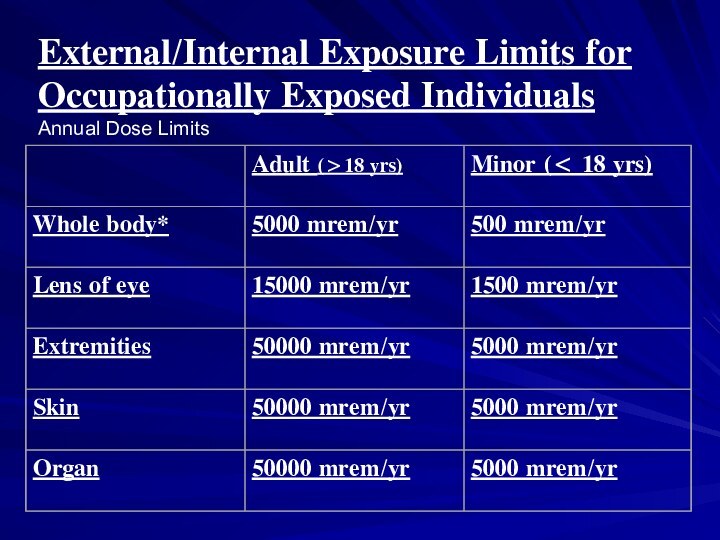
External/Internal Exposure Limits for Occupationally Exposed Individuals
Annual Dose
Limits
*Effective dose equivalent
Слайд 43
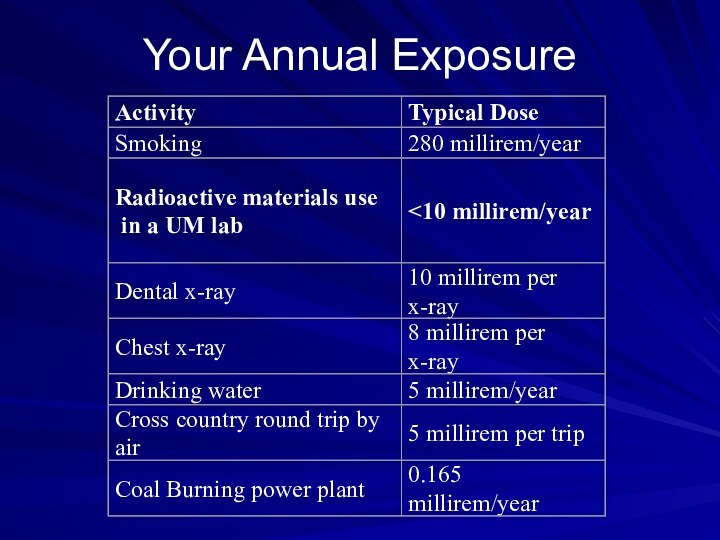
Community Emergency Radiation
Hazardous Waste Sites:
Radiation above background
(0.01-0.02 m rem/hr) signifies possible presence which must be
monitored. Radiation above 2 m rem/hr indicates potential hazard. Evacuate site until controlled.
Слайд 45
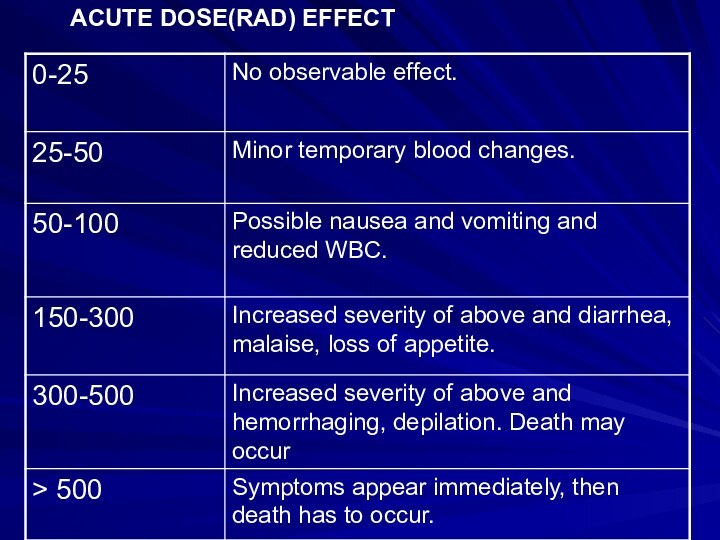
HEALTH EFFECTS
Generalizations: Biological effects are due to
the ionization process that destroys the capacity for cell
reproduction or division or causes cell mutation. A given total dose will cause more damage if received in a shorter time period. A fatal dose is (600 R)Acute Somatic Effects: Relatively immediate effects to a person acutely exposed. Severity depends on dose. Death usually results from damage to bone marrow or intestinal wall. Acute radio-dermatitis is common in radiotherapy; chronic cases occur mostly in industry.
Слайд 47
Delayed Somatic Effects: Delayed effects to exposed person
include: Cancer, leukemia, cataracts, life shortening from organ failure,
and abortion. Probability of an effect is proportional to dose (no threshold). Severity is independent of dose. Doubling dose for cancer is approximately 10-100 rems.Genetic Effects: Genetic effects to off-spring of exposed persons are irreversible and nearly always harmful. Doubling dose for mutation rate is approximately 50-80 rems. (Spontaneous mutation rate is approx. 10-100 mutations per million population per generation.)
Слайд 48
Critical Organs: Organs generally most susceptible to radiation
damage include: Lymphocytes, bone marrow, gastro-intestinal, gonads, and other
fast-growing cells. The central nervous system is relatively resistant. Many nuclides concentrate in certain organs rather than being uniformly distributed over the body, and the organs may be particularly sensitive to radiation damage, e.g., isotopes of iodine concentrate in the thyroid gland. These organs are considered "critical" for the specific nuclide.
Слайд 49
Non-ionizing Radiation
Definition:
“ They are electromagnetic waves incapable of
producing ions while passing through matter, due to their
lower energy.”
Слайд 50
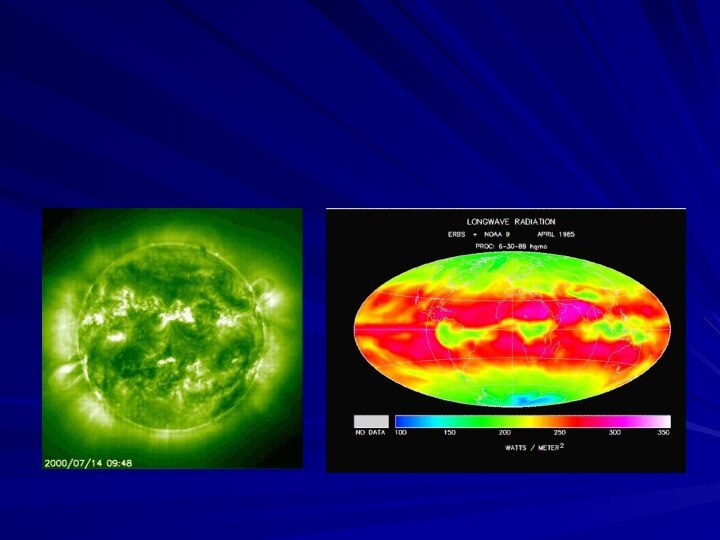
All earth surface system components emit radiation---the sun
and the earth are the components we are most
interested inThe sun emits radiation composed of high energy infrared radiation, visible light, and ultraviolet radiation collectively known as shortwave radiation (SW)
The earth emits radiation composed of lower energy infrared radiation collectively known as long-wave radiation (LW)
Слайд 54
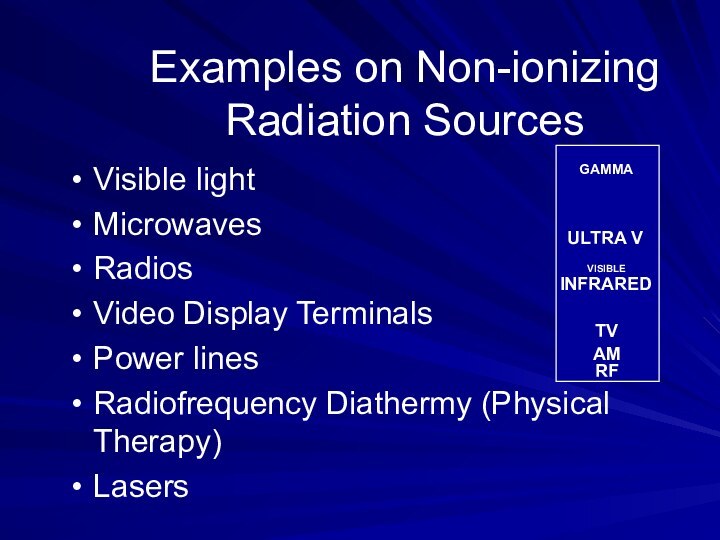
Examples on Non-ionizing Radiation Sources
Visible light
Microwaves
Radios
Video Display Terminals
Power
lines
Radiofrequency Diathermy (Physical Therapy)
Lasers
Слайд 58
Effects
Radiofrequency Ranges (10 kHz to 300 GHz)
Effects
only possible at ten times the permissible exposure limit
Heating
of the body (thermal effect)Cataracts
Some studies show effects of teratoginicity and carcinogenicity.
Слайд 59
RADIATION CONTROLS
A. Basic Control Methods for External Radiation
Decrease Time
Increase Distance
Increase Shielding
Слайд 60
Time: Minimize time of exposure to minimize total
dose. Rotate employees to restrict individual dose.
Distance: Maximize
distance to source to maximize attenuation in air. The effect of distance can be estimated from equations.Shielding: Minimize exposure by placing absorbing shield between worker and source.
Слайд 62
B. Monitoring
Personal Dosimeters: Normally they do not
prevent exposures (no alarm), just record it. They can
provide a record of accumulated exposure for an individual worker over extended periods of time (hours, days or weeks), and are small enough for measuring localized exposures Common types: Film badges; Thermoluminescence detectors (TLD); and pocket dosimeters.
Слайд 66
Direct Reading Survey Meters and Counters: Useful in
identifying source of exposures recorded by personal dosimeters, and
in evaluating potential sources, such as surface or sample contamination, source leakage, inadequate decontamination procedures, background radiation.Common types:
Alpha ? Proportional or Scintillation counters Beta, gamma ? Geiger-Mueller or Proportional counters X-ray, Gamma ? Ionization chambers Neutrons ? Proportional counters
Слайд 68
Continuous Monitors: Continuous direct reading ionization detectors (same
detectors as above) can provide read-out and/or alarm to
monitor hazardous locations and alert workers to leakage, thereby preventing exposures.Long-Term Samplers: Used to measure average exposures over a longer time period. For example, charcoal canisters or electrets are set out for days to months to measure radon in basements (should be <4 pCi/L).
Слайд 69
Elements of Radiation Protection Program
Monitoring of exposures:
Personal, area, and screening measurements; Medical/biologic monitoring.
Task-Specific Procedures
and Controls: Initial, periodic, and post-maintenance or other non-scheduled events. Engineering (shielding) vs. PPE vs. administrative controls. Including management and employee commitment and authority to enforce procedures and controls. Emergency procedures: Response, "clean-up", post clean-up testing and spill control.
Training and Hazard Communications including signs, warning lights, lockout/tagout, etc. Criteria for need, design, and information given.
Material Handling: Receiving, inventory control, storage, and disposal.





























