Слайд 2
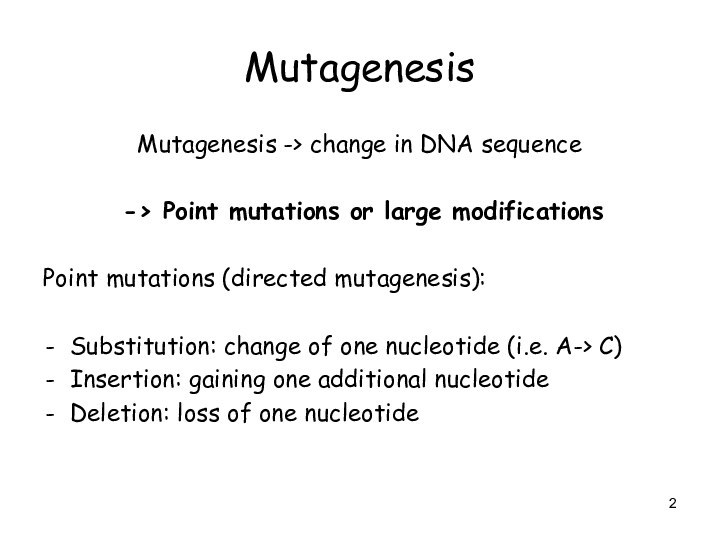
Mutagenesis
Mutagenesis -> change in DNA sequence
-> Point
mutations or large modifications
Point mutations (directed mutagenesis):
Substitution: change of
one nucleotide (i.e. A-> C)
Insertion: gaining one additional nucleotide
Deletion: loss of one nucleotide
Слайд 3
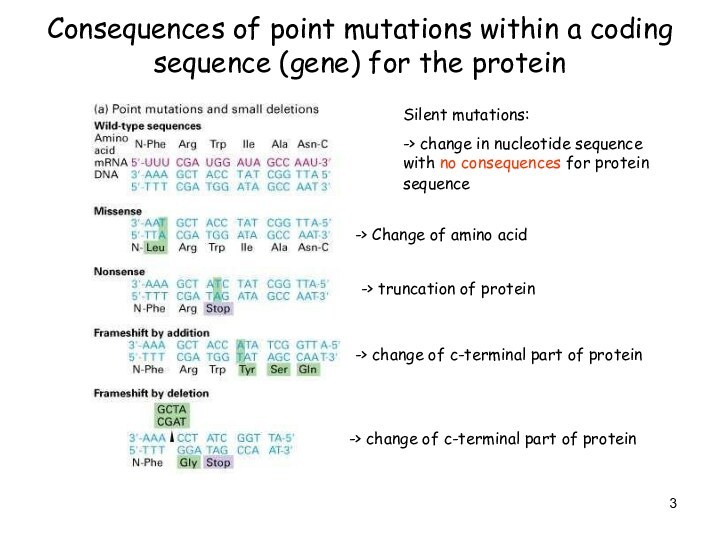
Consequences of point mutations within a coding sequence
(gene) for the protein
Silent mutations:
-> change in nucleotide sequence
with no consequences for protein sequence
-> Change of amino acid
-> truncation of protein
-> change of c-terminal part of protein
-> change of c-terminal part of protein
Слайд 4
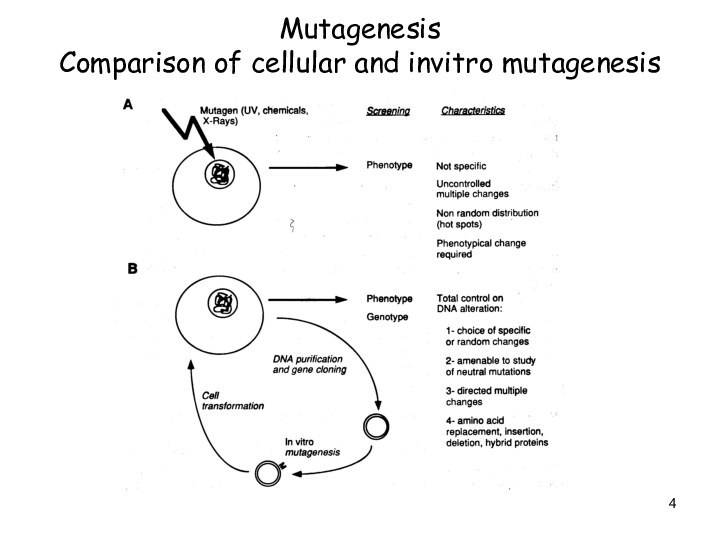
Mutagenesis
Comparison of cellular and invitro mutagenesis
Слайд 5
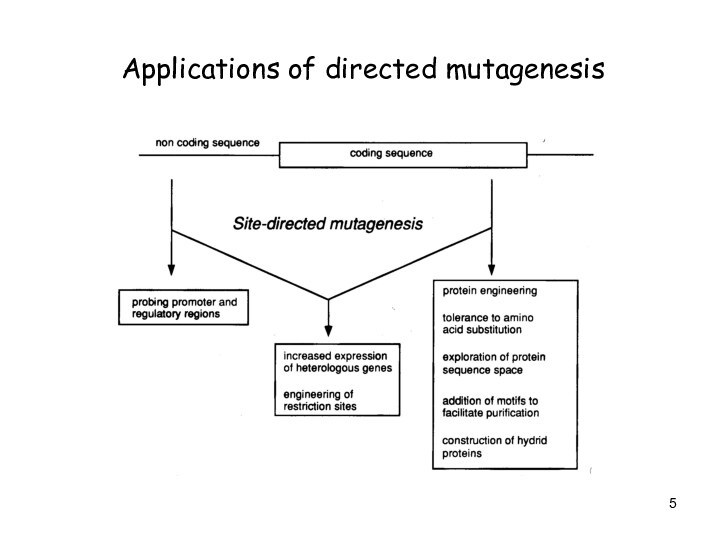
Applications of directed mutagenesis
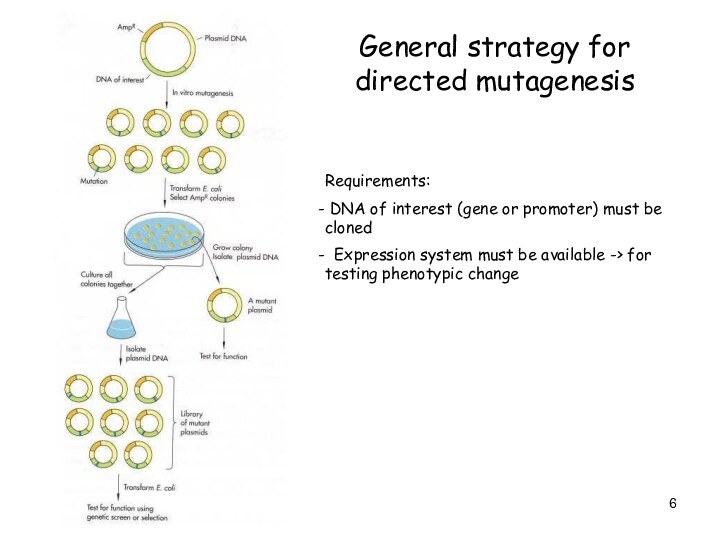
Слайд 6
General strategy for directed mutagenesis
Requirements:
DNA of
interest (gene or promoter) must be cloned
Expression
system must be available -> for testing phenotypic change
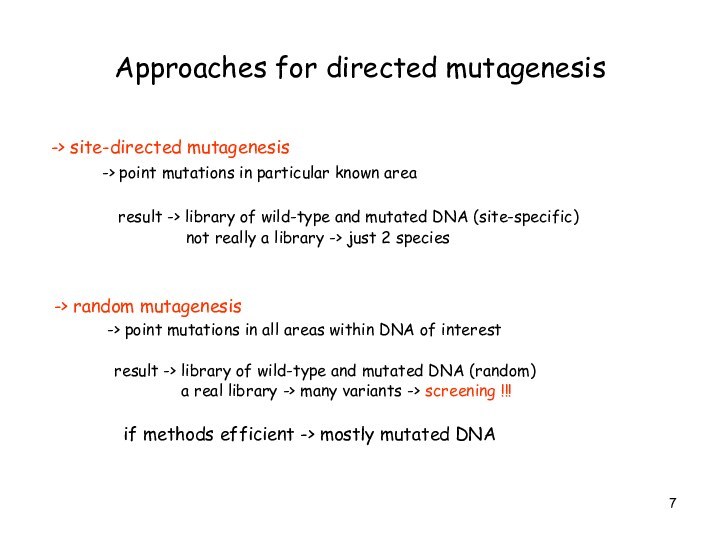
Слайд 7
Approaches for directed mutagenesis
-> site-directed mutagenesis
-> point mutations in particular
known area
result -> library of wild-type and mutated DNA (site-specific)
not really a library -> just 2 species
-> random mutagenesis
-> point mutations in all areas within DNA of interest
result -> library of wild-type and mutated DNA (random)
a real library -> many variants -> screening !!!
if methods efficient -> mostly mutated DNA
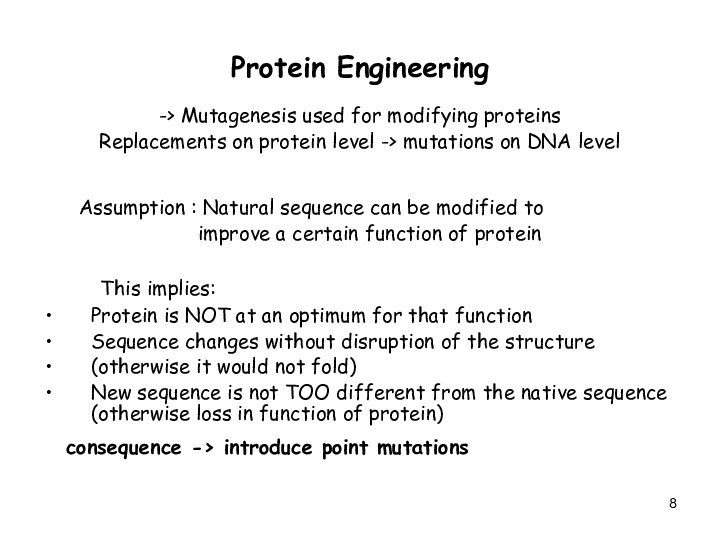
Слайд 8
Protein Engineering
-> Mutagenesis used for modifying proteins
Replacements on
protein level -> mutations on DNA level
Assumption : Natural sequence can be modified to
improve a certain function of protein
This implies:
Protein is NOT at an optimum for that function
Sequence changes without disruption of the structure
(otherwise it would not fold)
New sequence is not TOO different from the native sequence (otherwise loss in function of protein)
consequence -> introduce point mutations
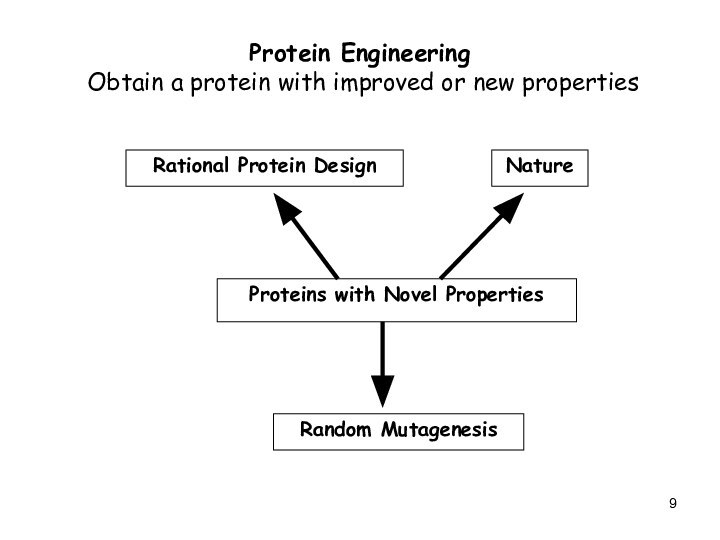
Слайд 9
Protein Engineering
Obtain a protein with improved
or new properties
Слайд 10
Rational Protein Design
Site –directed mutagenesis !!!
Requirements:
-> Knowledge of sequence and preferable Structure
(active site,….)
-> Understanding of mechanism
(knowledge about structure – function relationship)
-> Identification of cofactors……..
Слайд 11
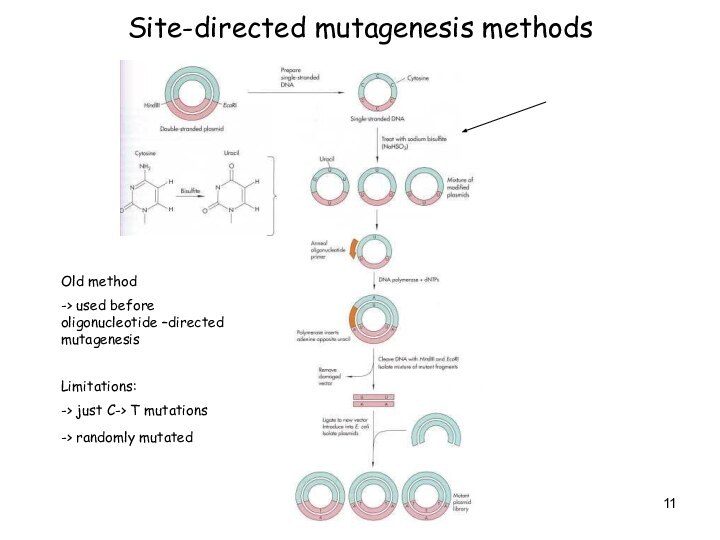
Site-directed mutagenesis methods
Old method
-> used before oligonucleotide
–directed mutagenesis
Limitations:
-> just C-> T mutations
-> randomly mutated
Слайд 12
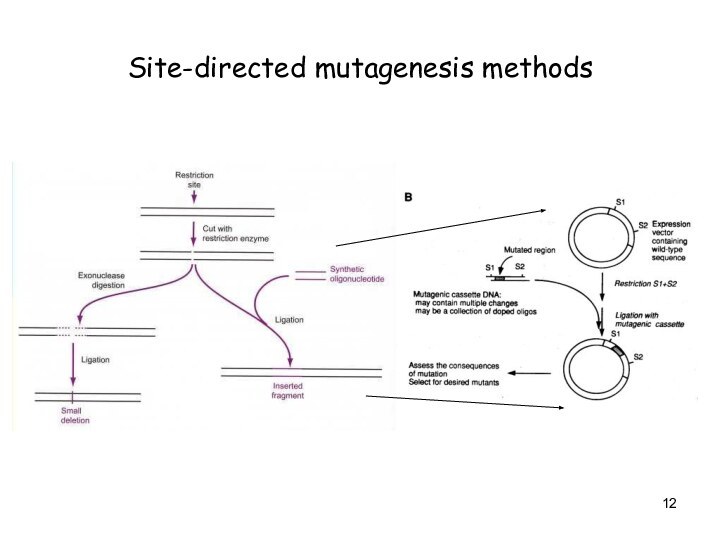
Site-directed mutagenesis methods
Слайд 13
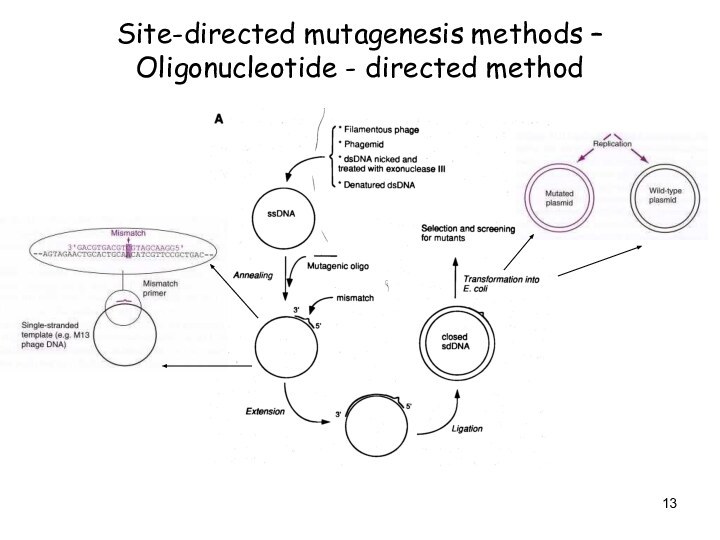
Site-directed mutagenesis methods – Oligonucleotide - directed method
Слайд 14
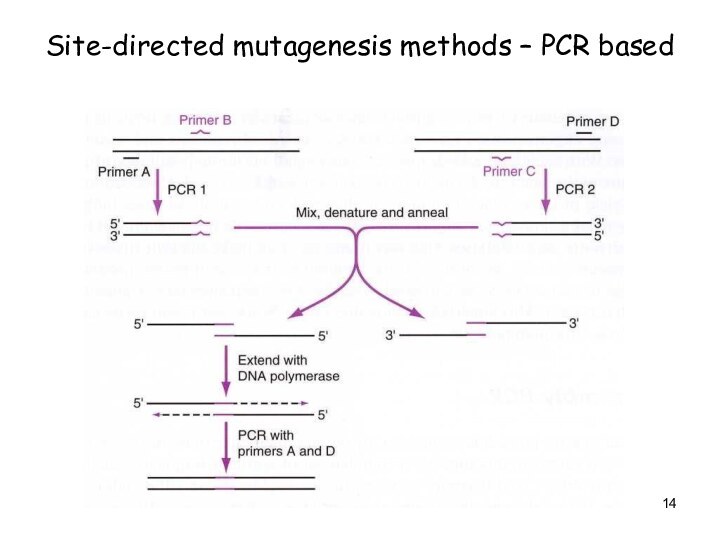
Site-directed mutagenesis methods – PCR based
Слайд 15
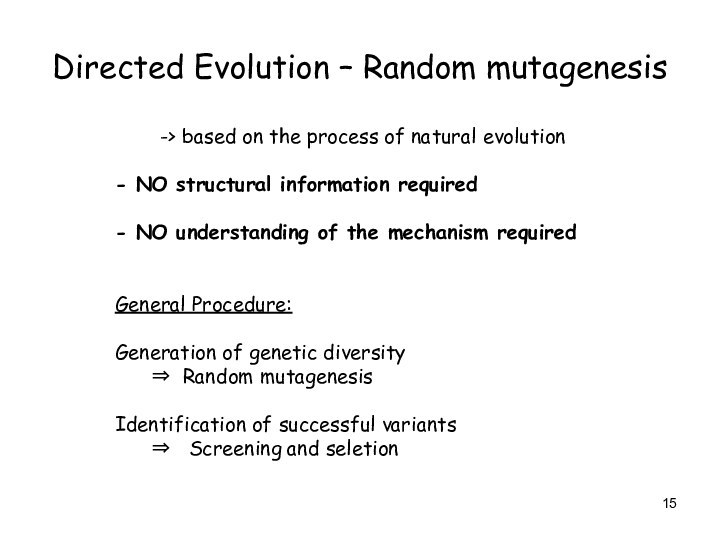
Directed Evolution – Random mutagenesis
-> based on the
process of natural evolution
- NO structural information required
- NO
understanding of the mechanism required
General Procedure:
Generation of genetic diversity
Random mutagenesis
Identification of successful variants
Screening and seletion
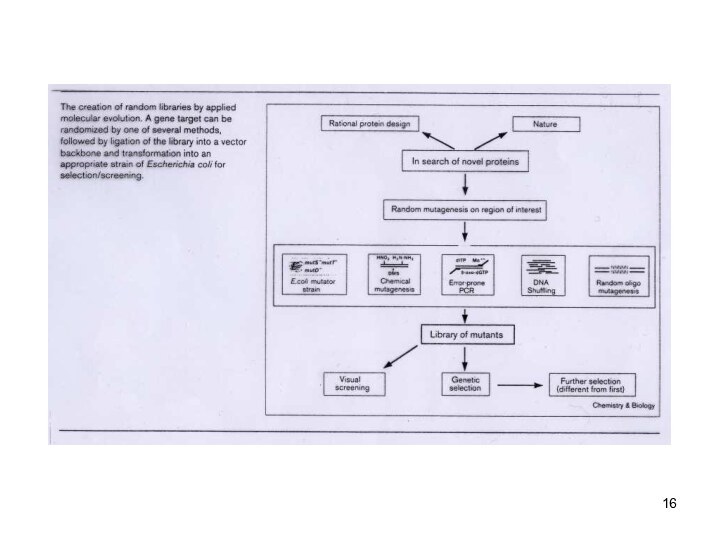
Слайд 17
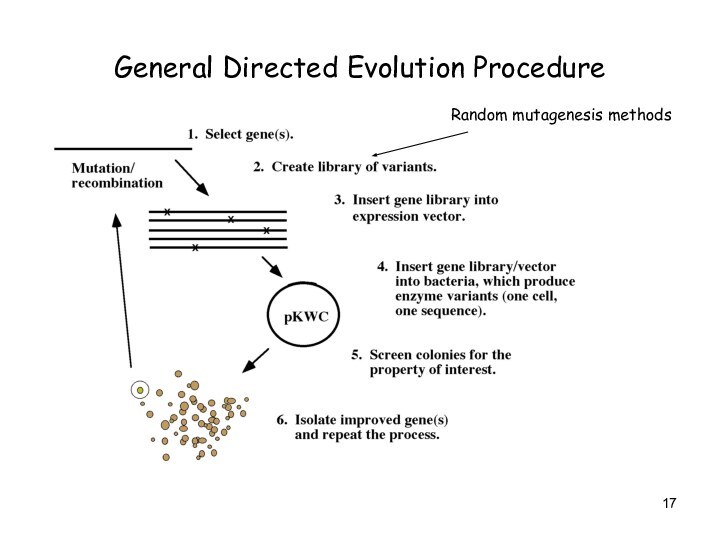
General Directed Evolution Procedure
Random mutagenesis methods
Слайд 18
Directed Evolution Library
Even a large library -> (108
independent clones)
will not exhaustively encode all possible single
point mutations.
Requirements would be:
20N independend clones -> to have all possible variations in a library
(+ silent mutations)
N….. number of amino acids in the protein
For a small protein: -> Hen egg-white Lysozyme (129 aa; 14.6 kDa)
-> library with 20129 (7x 10168) independent clones
Consequence -> not all modifications possible
-> modifications just along an evolutionary path !!!!
Слайд 19
Limitation of Directed Evolution
Evolutionary path must exist -
> to be successful
Screening method must be available
-> You get (exactly) what you ask for!!!
-> need to be done in -> High throughput !!!
Слайд 20
Successful experiments involve generally
less than 6 steps
(cycles)!!!
Why?
Sequences with improved properties are rather close to the
parental sequence -> along a evolutionary path
2. Capacity of our present methods to generate novel functional sequences is rather limited -> requires huge libraries
Point Mutations !!!
Typical Directed Evolution Experiment
Слайд 21
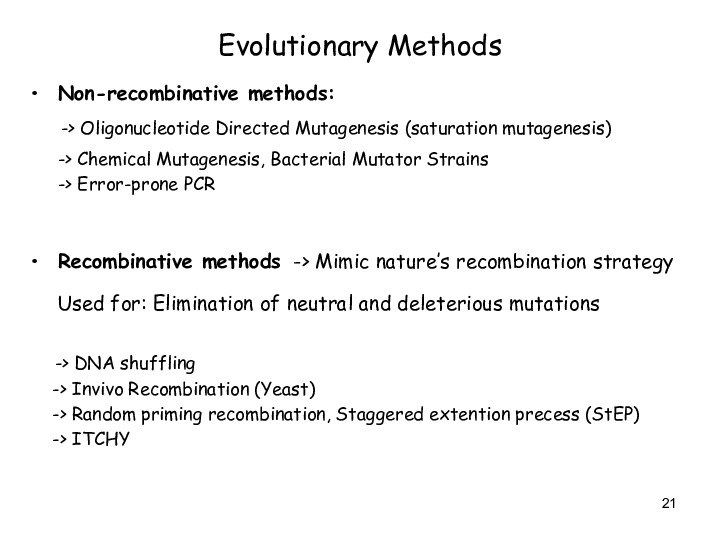
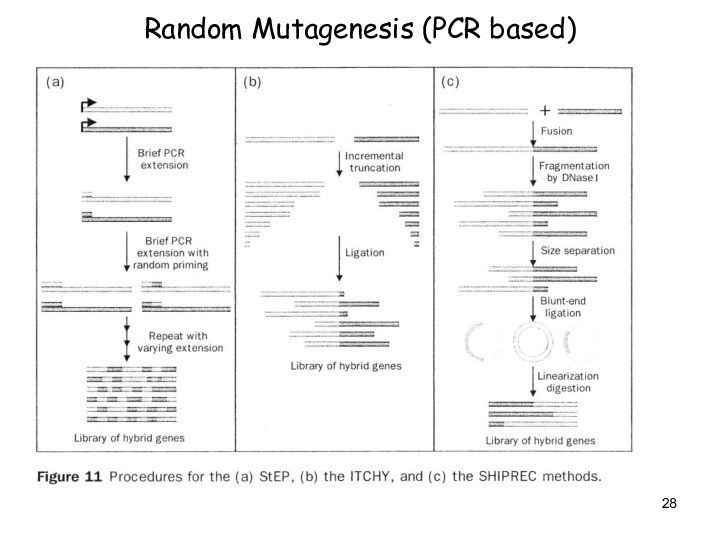
Evolutionary Methods
Non-recombinative methods:
-> Oligonucleotide Directed
Mutagenesis (saturation mutagenesis)
-> Chemical Mutagenesis, Bacterial
Mutator Strains
-> Error-prone PCR
Recombinative methods -> Mimic nature’s recombination strategy
Used for: Elimination of neutral and deleterious mutations
-> DNA shuffling
-> Invivo Recombination (Yeast)
-> Random priming recombination, Staggered extention precess (StEP)
-> ITCHY
Слайд 22
Evolutionary Methods
Type of mutation – Fitness of mutants
Type
of mutations:
Beneficial mutations (good)
Neutral mutations
Deleterious mutations (bad)
Beneficial mutations are
diluted with neutral and deleterious ones
!!! Keep the number of mutations low per cycle
-> improve fitness of mutants!!!
Слайд 23
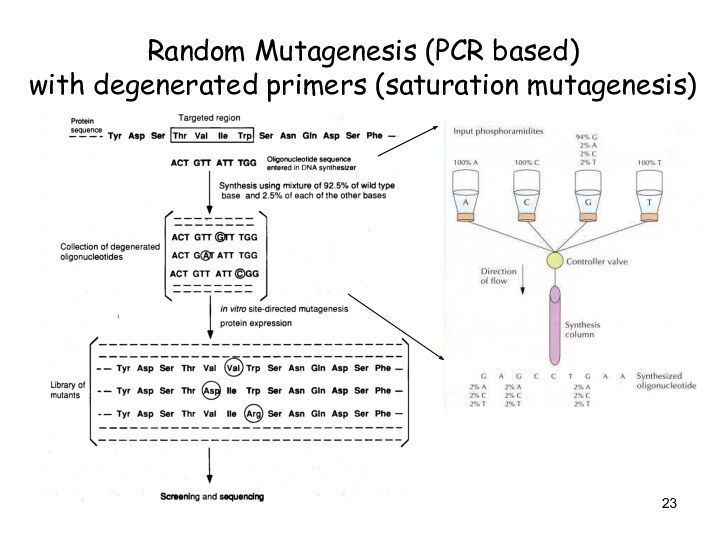
Random Mutagenesis (PCR based)
with degenerated primers (saturation
mutagenesis)
Слайд 24
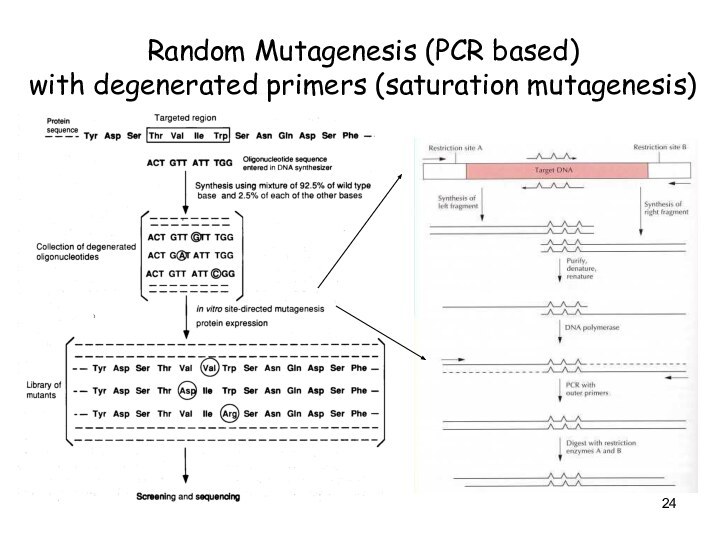
Random Mutagenesis (PCR based)
with degenerated primers (saturation
mutagenesis)
Слайд 25
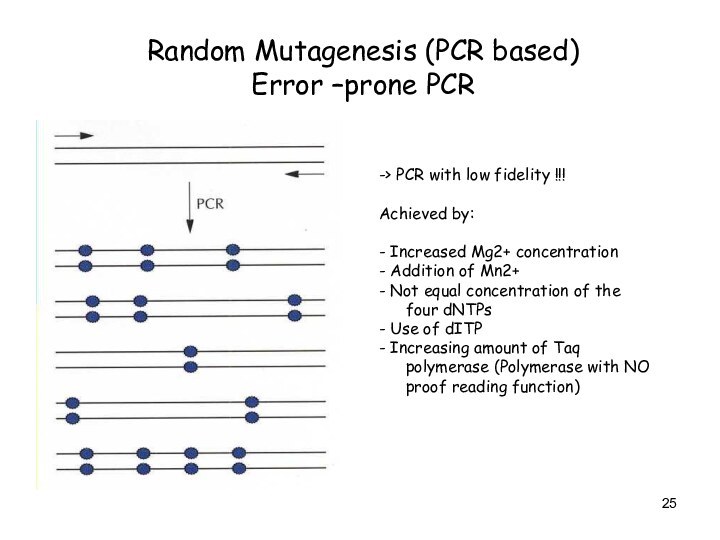
Random Mutagenesis (PCR based)
Error –prone PCR
-> PCR
with low fidelity !!!
Achieved by:
- Increased Mg2+ concentration
- Addition
of Mn2+
- Not equal concentration of the four dNTPs
- Use of dITP
- Increasing amount of Taq polymerase (Polymerase with NO proof reading function)
Слайд 26
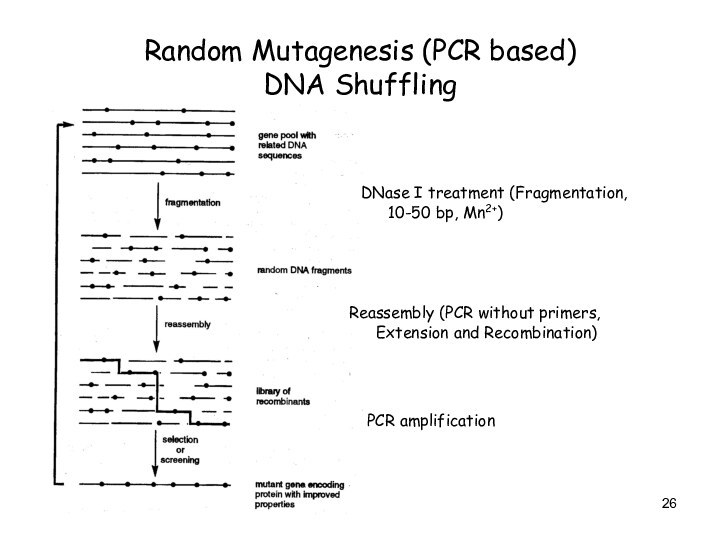
Random Mutagenesis (PCR based)
DNA Shuffling
DNase I treatment
(Fragmentation, 10-50 bp, Mn2+)
Reassembly (PCR without primers, Extension and
Recombination)
PCR amplification
Слайд 27
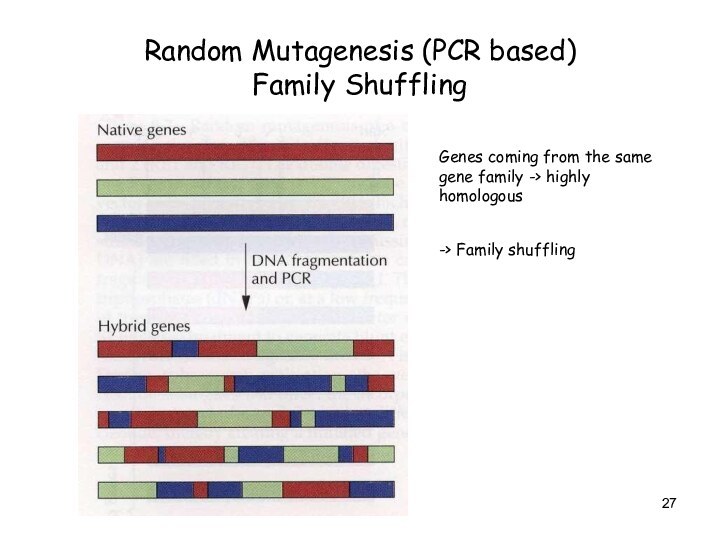
Random Mutagenesis (PCR based)
Family Shuffling
Genes coming from
the same gene family -> highly homologous
-> Family shuffling
Слайд 29
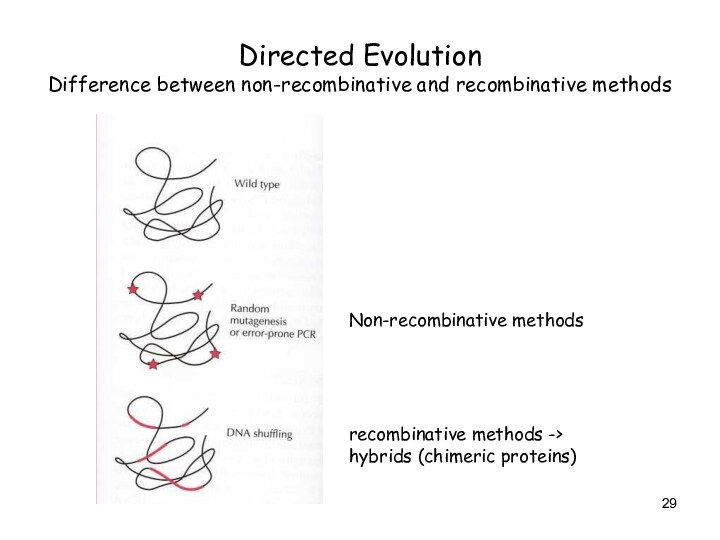
Directed Evolution
Difference between non-recombinative and recombinative methods
Non-recombinative methods
recombinative
methods -> hybrids (chimeric proteins)
Слайд 30
Protein Engineering
What can be engineered in Proteins ?
->
Folding (+Structure):
1. Thermodynamic Stability
(Equilibrium between: Native
Unfolded state)
2. Thermal and Environmental Stability (Temperature, pH, Solvent, Detergents, Salt …..)
Слайд 31
Protein Engineering
What can be engineered in Proteins ?
->
Function:
1. Binding (Interaction of a protein with its surroundings)
How
many points are required to bind a molecule with high affinity?
Catalysis (a different form of binding – binding the transition state of a chemical reaction)
Increased binding to the transition state increased catalytic rates !!!
Requires: Knowledge of the Catalytic Mechanism !!!
-> engineer Kcat and Km
Слайд 32
Protein Engineering
Factors which contribute to stability:
Hydrophobicity (hydrophobic
core)
Electrostatic Interactions:
-> Salt Bridges
-> Hydrogen Bonds
-> Dipole Interactions
Disulfide Bridges
Metal Binding (Metal chelating site)
Reduction of the unfolded state entropy with
X Pro mutations
Слайд 33
Protein Engineering
Design of Thermal and Environmental stability:
Stabilization
of -Helix Macrodipoles
Engineer Structural Motifes (like Helix N-Caps)
Introduction of salt bridges
Introduction of residues with higher intrinsic properties for their conformational state (e.g. Ala replacement within a -Helix)
Introduction of disulfide bridges
Reduction of the unfolded state entropy with
X Pro mutations
Слайд 34
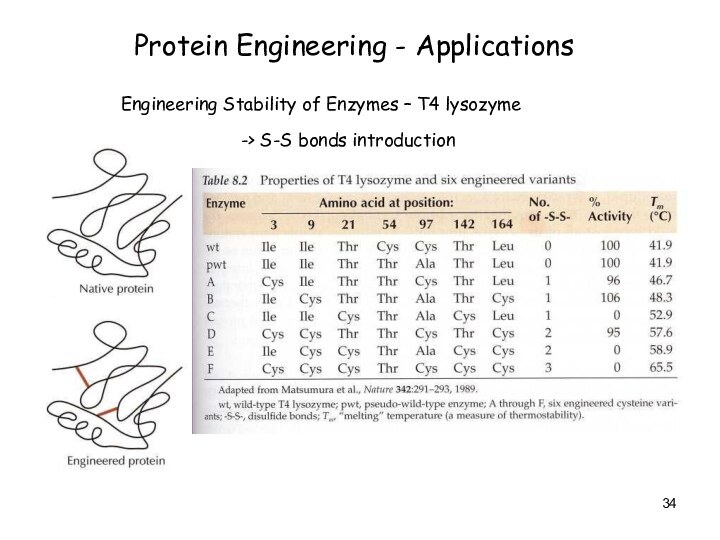
Protein Engineering - Applications
Engineering Stability of Enzymes –
T4 lysozyme
-> S-S bonds introduction
Слайд 35
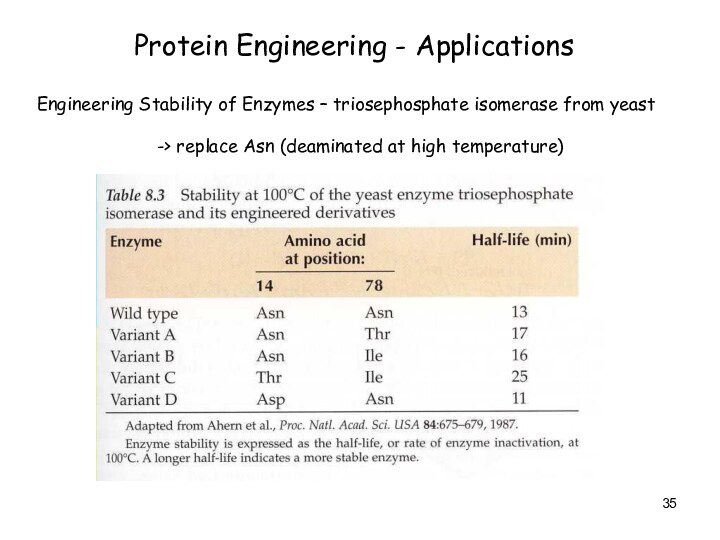
Protein Engineering - Applications
Engineering Stability of Enzymes –
triosephosphate isomerase from yeast
-> replace Asn (deaminated at high
temperature)
Слайд 36
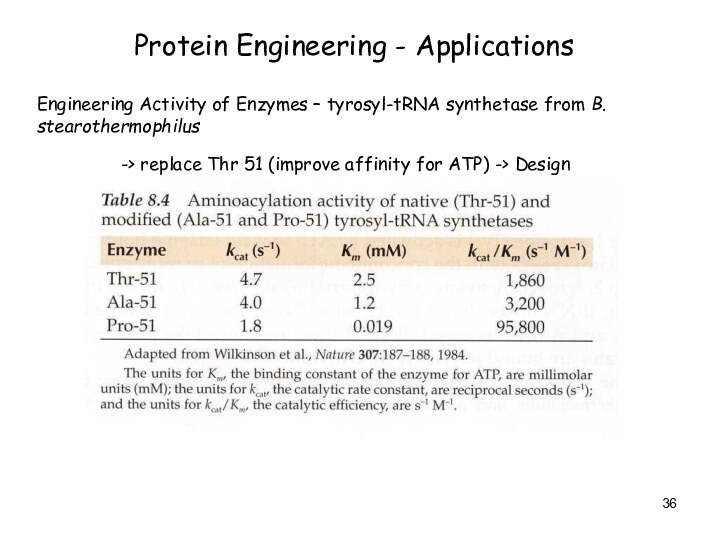
Protein Engineering - Applications
Engineering Activity of Enzymes –
tyrosyl-tRNA synthetase from B. stearothermophilus
-> replace Thr 51 (improve
affinity for ATP) -> Design
Слайд 37
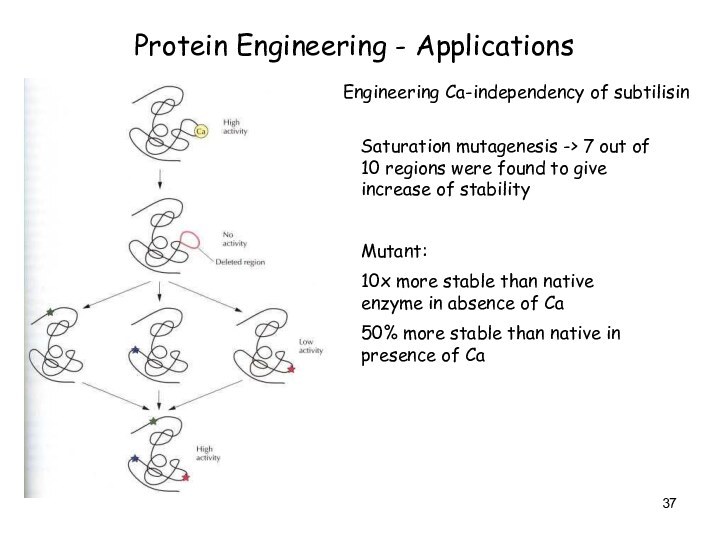
Protein Engineering - Applications
Engineering Ca-independency of subtilisin
Saturation mutagenesis
-> 7 out of 10 regions were found to
give increase of stability
Mutant:
10x more stable than native enzyme in absence of Ca
50% more stable than native in presence of Ca
Слайд 38
DNA shuffling
JCohen. News note: How DNA
shuffling works. Sci 293:237 (2001)
Maxygen, PCR without synthetic primers
Using
family of related genes, digest into fragments
Heat and renature randomly
Use as PCR primers
Слайд 39
Altering multiple properties: rapid high-throughput screening
ex.,
subtilisin
Use 26 different subtilisin genes
Shuffle DNA, construct library of
654 clones, and Tf B. subtilis
Assay in microtiter plates: originals plus clones
Activity at 23C; thermostability; solvent stability; pH dependence
Of 654 clones, 77 versions performed as well as or better than parents at 23C
Sequencing showed chimeras; one has 8 crossovers with 15 AAc substitutions
Слайд 40
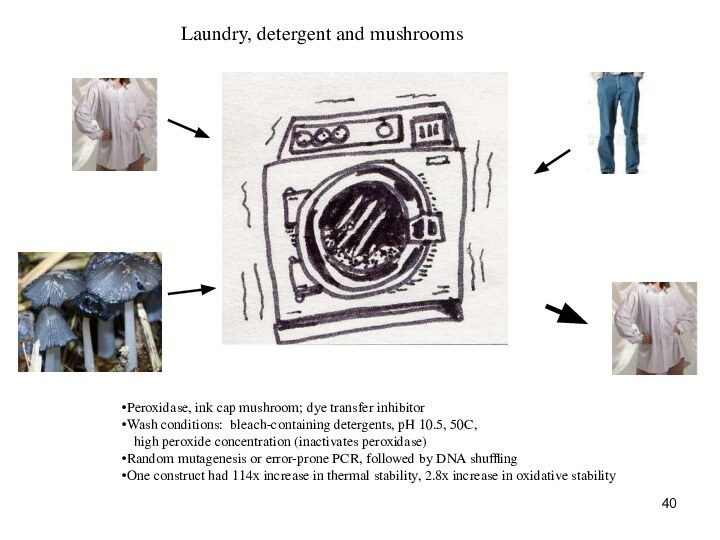
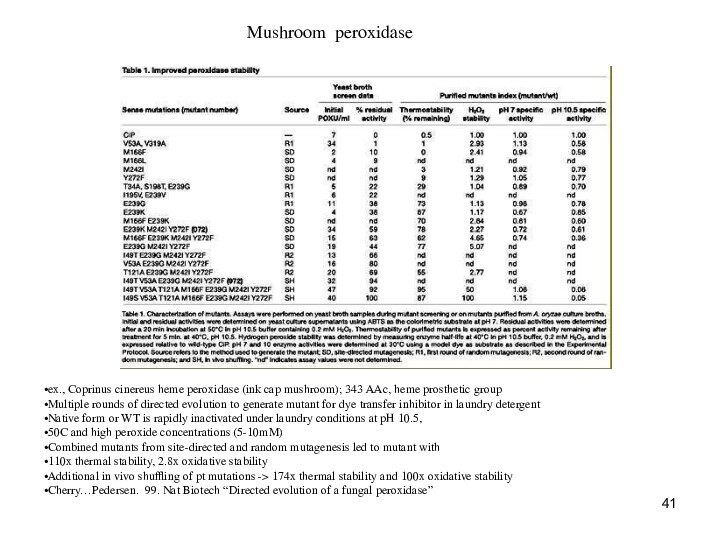
Laundry, detergent and mushrooms
Peroxidase, ink cap mushroom;
dye transfer inhibitor
Wash conditions: bleach-containing detergents, pH 10.5, 50C,
high peroxide concentration (inactivates peroxidase)
Random mutagenesis or error-prone PCR, followed by DNA shuffling
One construct had 114x increase in thermal stability, 2.8x increase in oxidative stability
Слайд 41
ex., Coprinus cinereus heme peroxidase (ink cap
mushroom); 343 AAc, heme prosthetic group
Multiple rounds of directed
evolution to generate mutant for dye transfer inhibitor in laundry detergent
Native form or WT is rapidly inactivated under laundry conditions at pH 10.5,
50C and high peroxide concentrations (5-10mM)
Combined mutants from site-directed and random mutagenesis led to mutant with
110x thermal stability, 2.8x oxidative stability
Additional in vivo shuffling of pt mutations -> 174x thermal stability and 100x oxidative stability
Cherry…Pedersen. 99. Nat Biotech “Directed evolution of a fungal peroxidase”
Mushroom peroxidase
Слайд 42
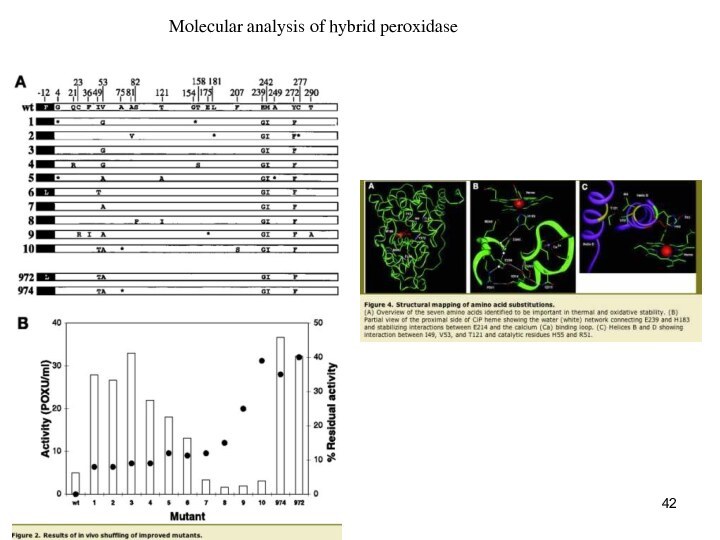
Molecular analysis of hybrid peroxidase
Слайд 43
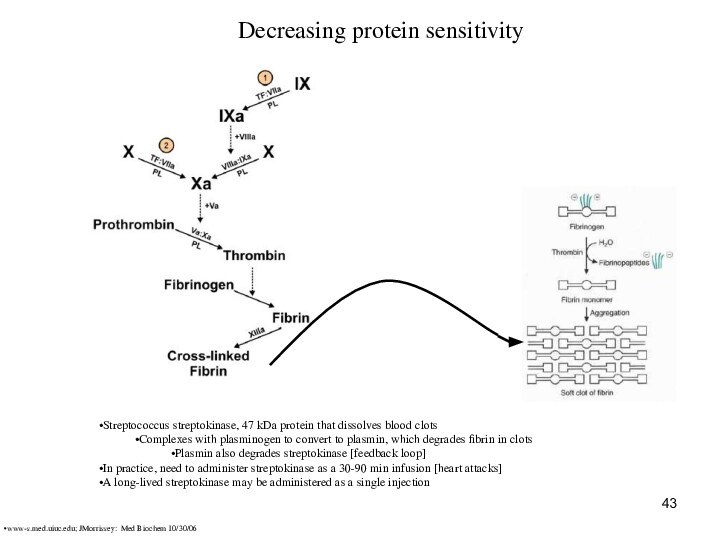
Decreasing protein sensitivity
Streptococcus streptokinase, 47 kDa protein
that dissolves blood clots
Complexes with plasminogen to convert to
plasmin, which degrades fibrin in clots
Plasmin also degrades streptokinase [feedback loop]
In practice, need to administer streptokinase as a 30-90 min infusion [heart attacks]
A long-lived streptokinase may be administered as a single injection
www-s.med.uiuc.edu; JMorrissey: Med Biochem 10/30/06
Слайд 44
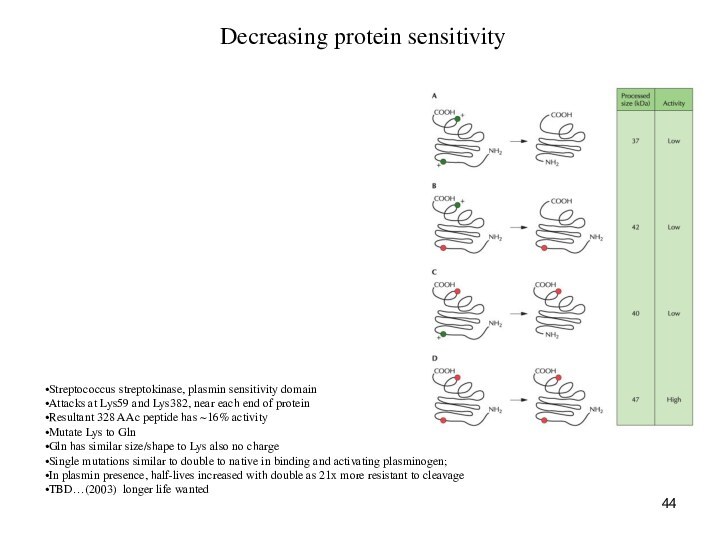
Decreasing protein sensitivity
Streptococcus streptokinase, plasmin sensitivity domain
Attacks
at Lys59 and Lys382, near each end of protein
Resultant
328 AAc peptide has ~16% activity
Mutate Lys to Gln
Gln has similar size/shape to Lys also no charge
Single mutations similar to double to native in binding and activating plasminogen;
In plasmin presence, half-lives increased with double as 21x more resistant to cleavage
TBD…(2003) longer life wanted
Слайд 45
Protein Engineering - Applications
Site-directed mutagenesis -> used to
alter a single property
Problem : changing one property ->
disrupts another characteristics
Directed Evolution (Molecular breeding) -> alteration of multiple properties
Слайд 46
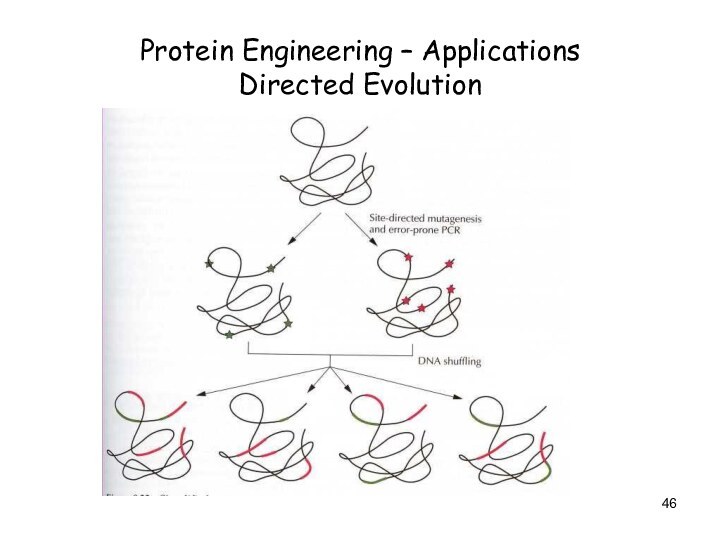
Protein Engineering – Applications
Directed Evolution
Слайд 47
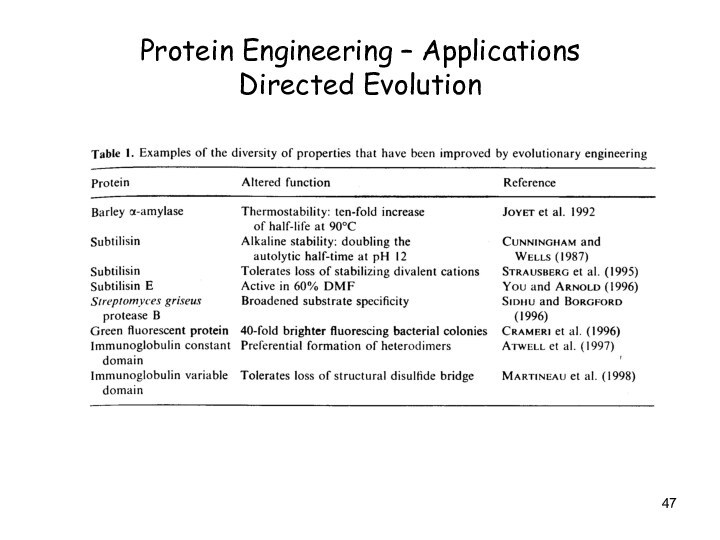
Protein Engineering – Applications
Directed Evolution
Слайд 48
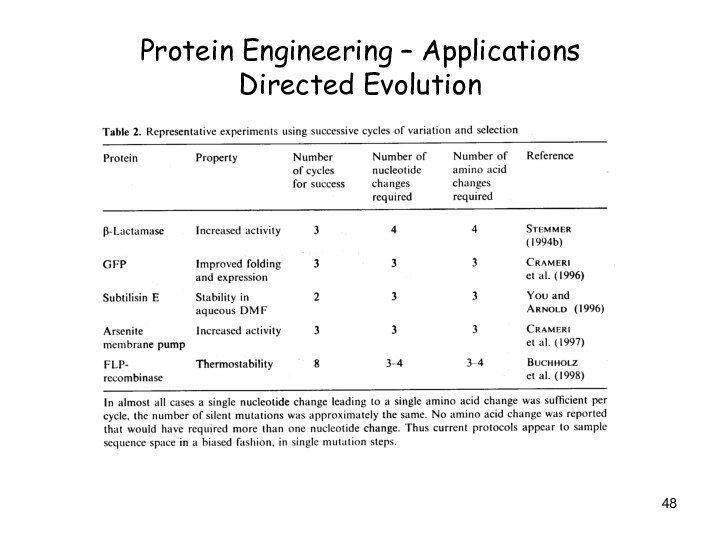
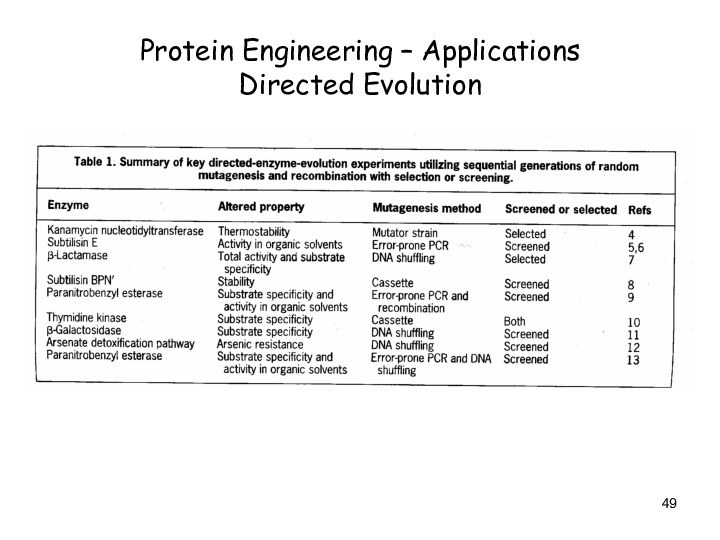
Protein Engineering – Applications
Directed Evolution
Слайд 49
Protein Engineering – Applications
Directed Evolution
Слайд 50
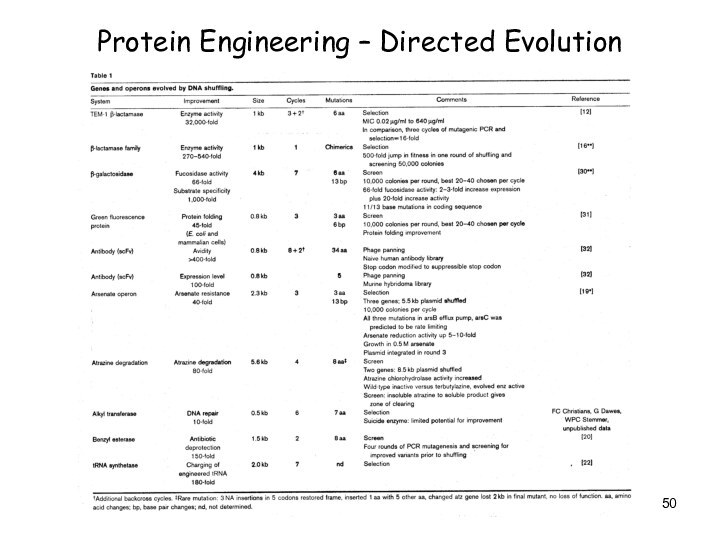
Protein Engineering – Directed Evolution
Слайд 51
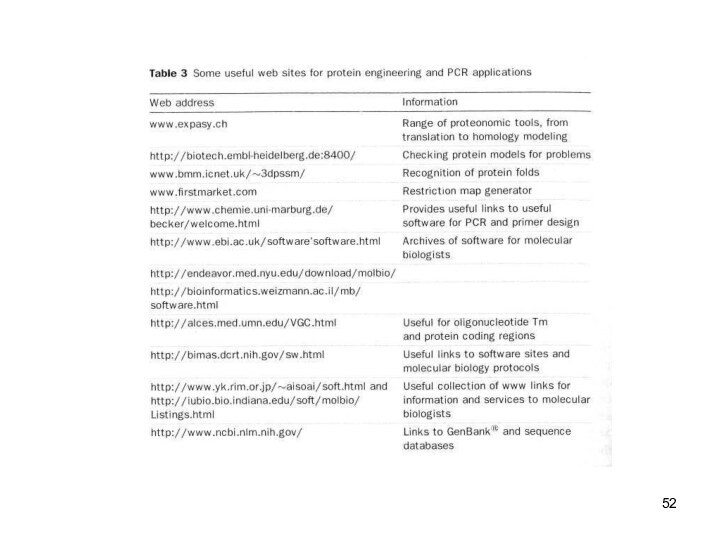
Protein Engineering - Applications