- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Biochemical reaction kinetics
Содержание
- 2. Chemical kinetics studies the rate and mechanism of chemical reactions
- 3. In homogeneous reactions all the reactants
- 4. Single-stage reactions are called simple (or elementary)
- 5. The dependence of the reaction rate on
- 6. Mathematical expression of the law of mass
- 7. Molecularity of the reaction is determined by
- 8. Arrenius Equation establishes a connection
- 9. Catalysis is the change of chemical reactions
- 10. Скачать презентацию
- 11. Похожие презентации
Chemical kinetics studies the rate and mechanism of chemical reactions





Слайд 3 In homogeneous reactions all the reactants exist in
the same phase in which the reaction itself occurs.
Na2CO3 + HCl ↔ NaHCO3 + NaCl Heterogeneous reactions take place only in the interphase. Fe + HCl⭢FeCl2 +H2
Слайд 4
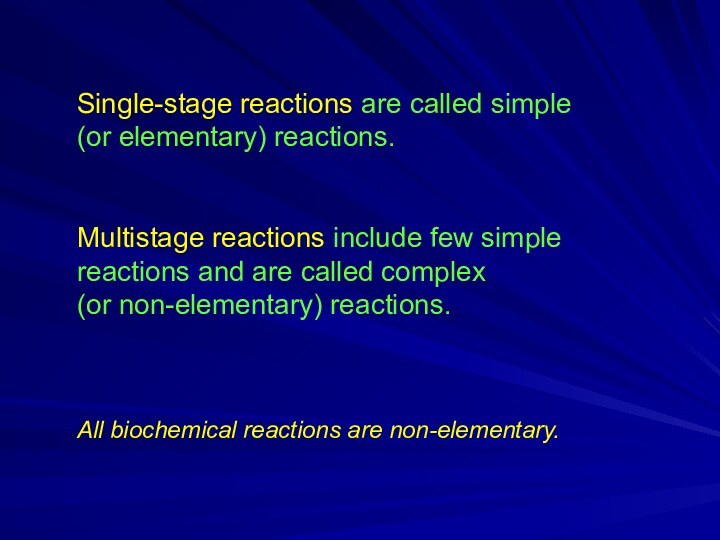
Single-stage reactions are called simple
(or elementary) reactions.
Multistage reactions include few simple reactions and are called
complex (or non-elementary) reactions.
All biochemical reactions are non-elementary.
Слайд 5 The dependence of the reaction rate on the
concentration of reactants is described by the law of
mass action discovered by N.Beketov, C. Guldberg and P. Waage in 1967:«At constant temperature the rate of chemical reaction is in direct proportion to the product of reactant concentrations in the degree of their stoichiometric coefficients».
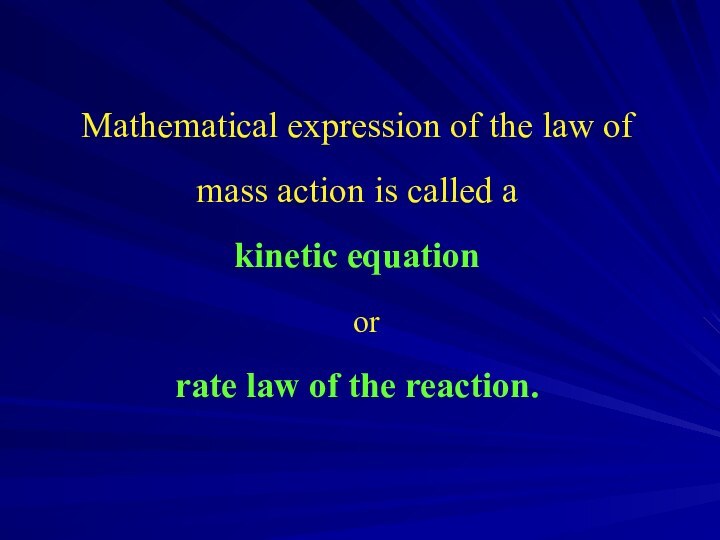
Слайд 6 Mathematical expression of the law of mass action
is called a
kinetic equation
or
rate law of
the reaction.
Слайд 7
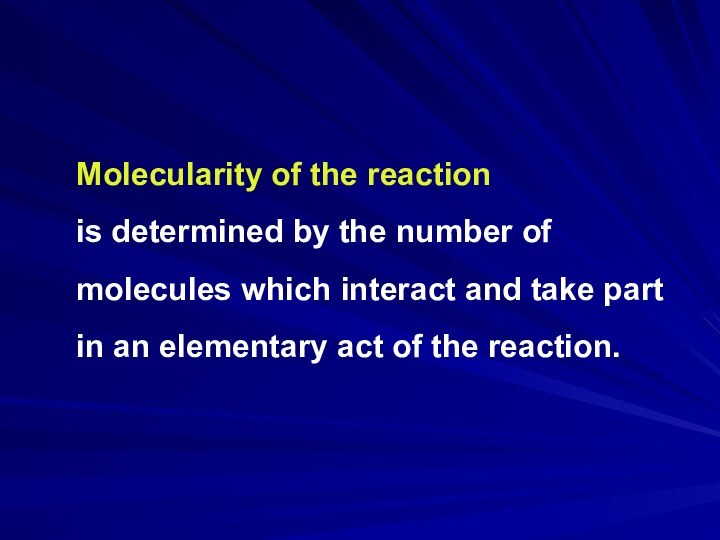
Molecularity of the reaction
is determined by the
number of molecules which interact and take part in
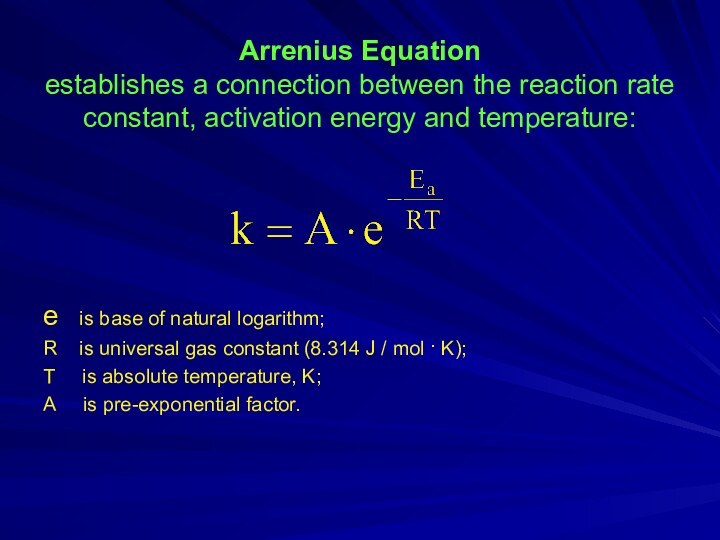
an elementary act of the reaction.Слайд 8 Arrenius Equation establishes a connection between the reaction
rate constant, activation energy and temperature:
e is base
of natural logarithm;R is universal gas constant (8.314 J / mol · K);
T is absolute temperature, K;
A is pre-exponential factor.
Слайд 9 Catalysis is the change of chemical reactions rate
under the influence of substances, the amount and nature
of which, after completion of the reaction are the same as before the reaction.Catalyst is a substance that influences the rate of chemical processes without changing its own chemical composition.