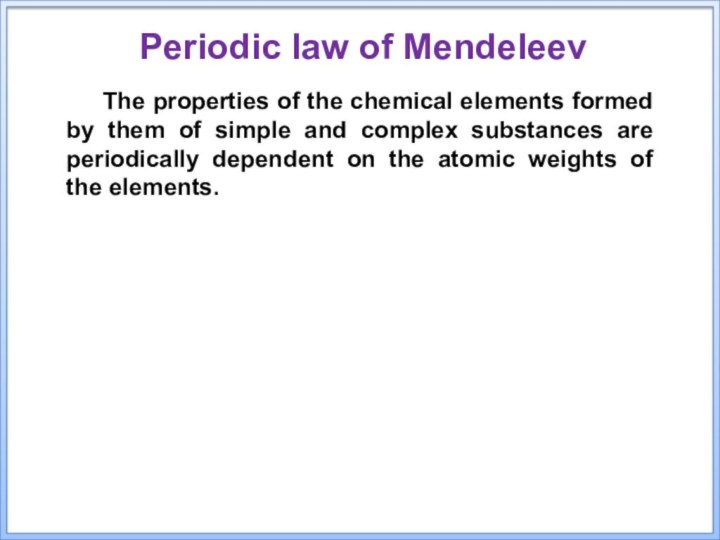
the 150th anniversary of the publication of the periodic
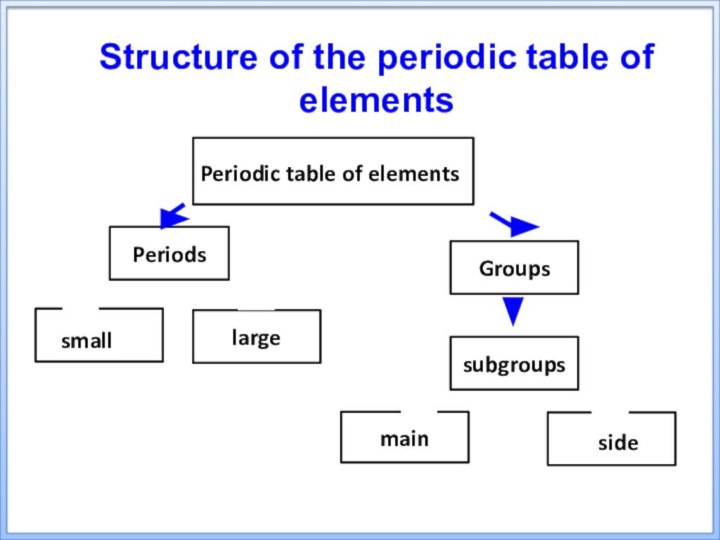
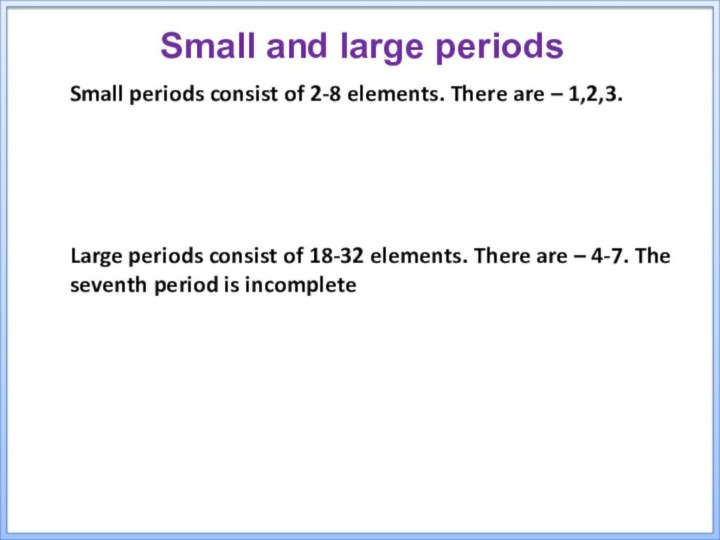
tableТhe purpose of the presentation: to acquaint students with the structure and laws of the periodic system
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Email: Нажмите что бы посмотреть













scientist's estate
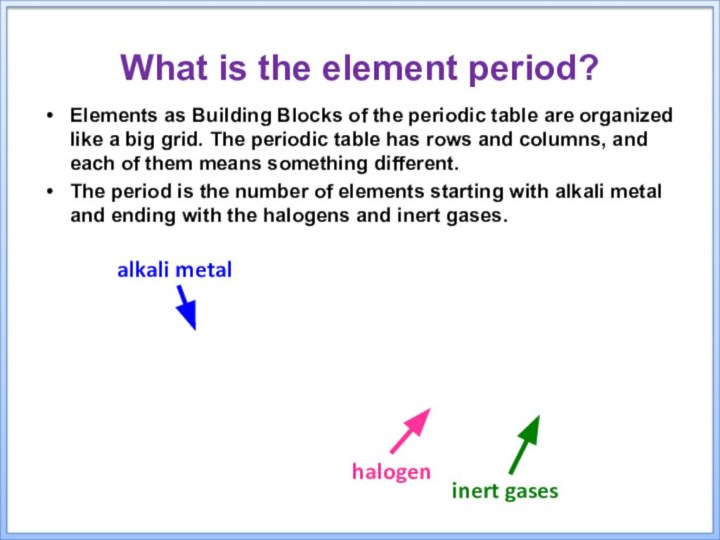
alkali metal
halogen
inert gases
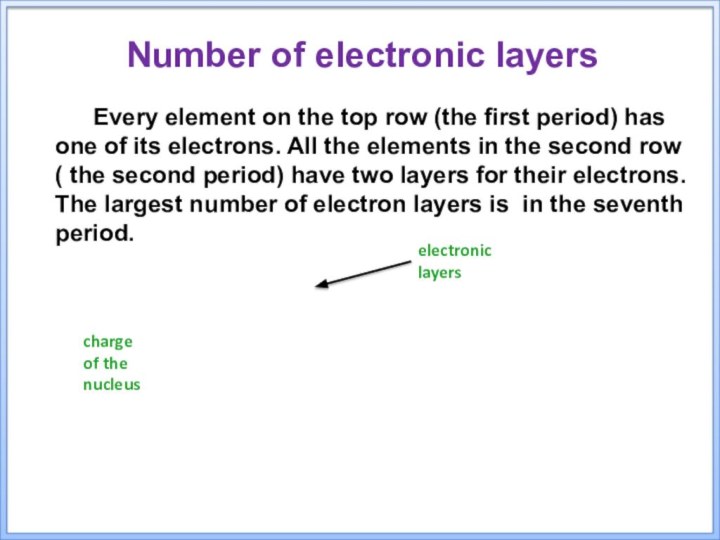
electronic layers
charge of the nucleus
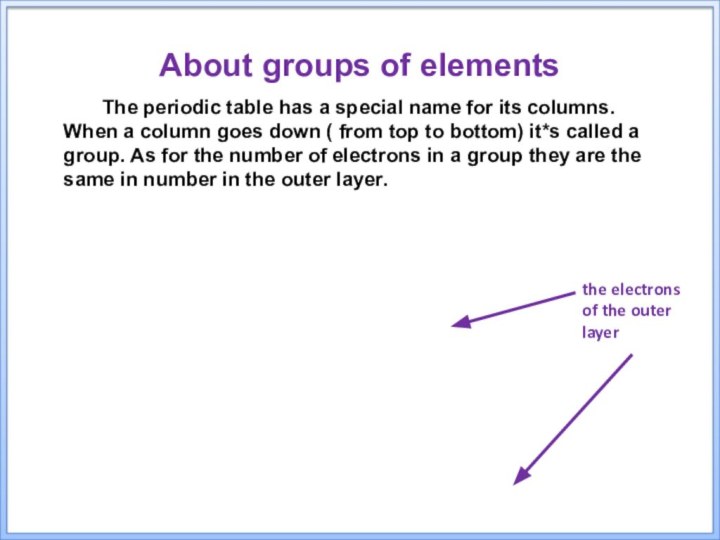
the electrons of the outer layer
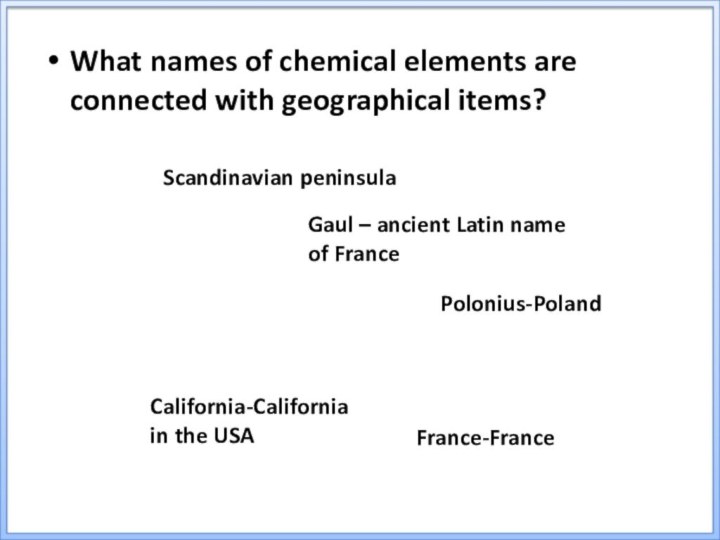
France-France