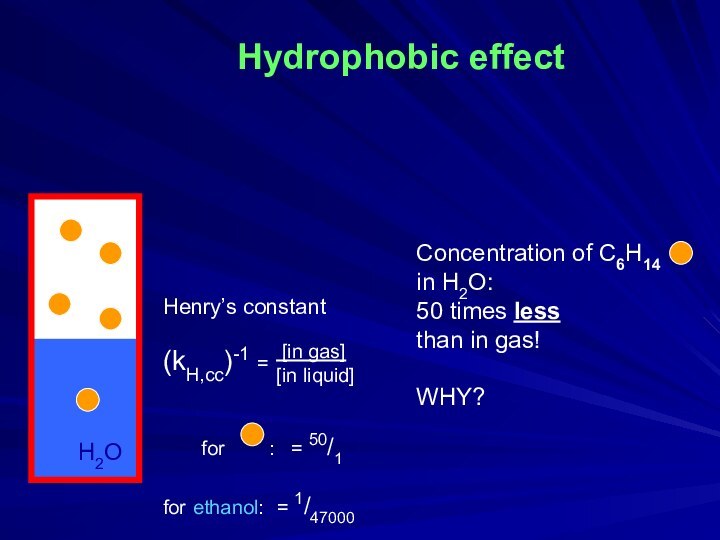
times less
than in gas!
WHY?
H2O
Henry’s constant
(kH,cc)-1 =
for : = 50/1
for ethanol: = 1/47000
[in gas]
[in liquid]
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Email: Нажмите что бы посмотреть
![Elementary interactions: hydrophobic & electrostatic; SS and coordinate bonds ENTROPY:SE = kB • ln[ME]; ME=number_of_states(E)Why kB? What is kB?Because](/img/tmb/15/1439696/467bdf22b303ef2ca7331d618dfff680-720x.jpg)
![Elementary interactions: hydrophobic & electrostatic; SS and coordinate bonds Experiment: ΔG intA→B= kBT•ln([C1 in A]/[C1 in B])ΔSintA→B = -d(ΔGintA→B)/dTΔHintA→B = ΔGintA→B](/img/tmb/15/1439696/c6caa6bf654fb75e4ea31d6c3759f9ab-720x.jpg)












![Elementary interactions: hydrophobic & electrostatic; SS and coordinate bonds Debye-Hückel screening of electrostatic by ions:U = [q1q2/εr]•exp(-r/D) ;](/img/tmb/15/1439696/8c2f89ac5188ee57c8d350370b34ca76-720x.jpg)

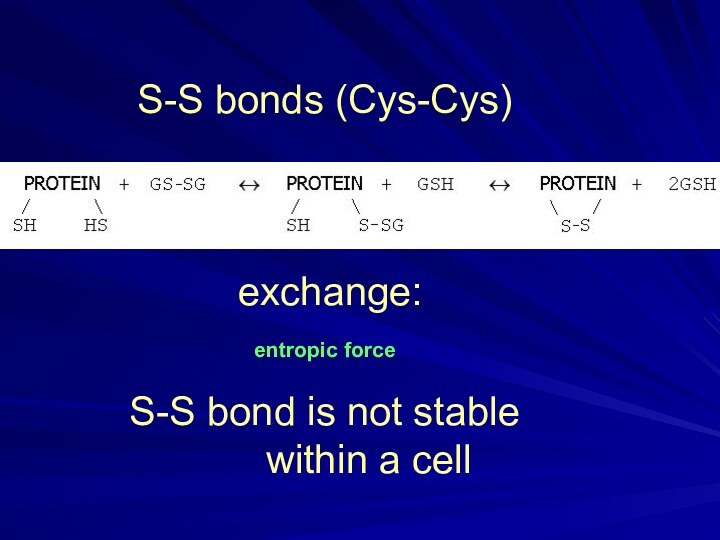
[in gas]
[in liquid]
T=2980K=250C
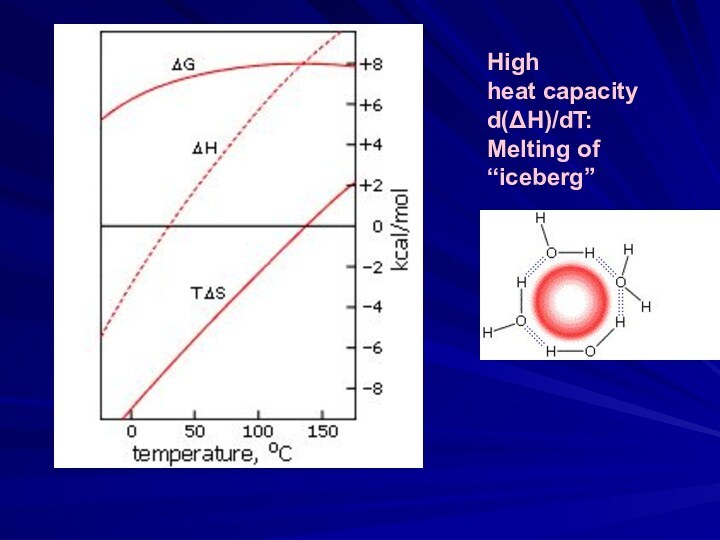
Hydrophobic
effect
&
denaturationof proteins
Charles Tanford
(1921 - 2009)
General physical
features of
Hydrophobic
effect
CHARGE
inside
PROTEIN
Water => vacuum:
ΔU ≈ +100 kcal/mol
-
- -
-
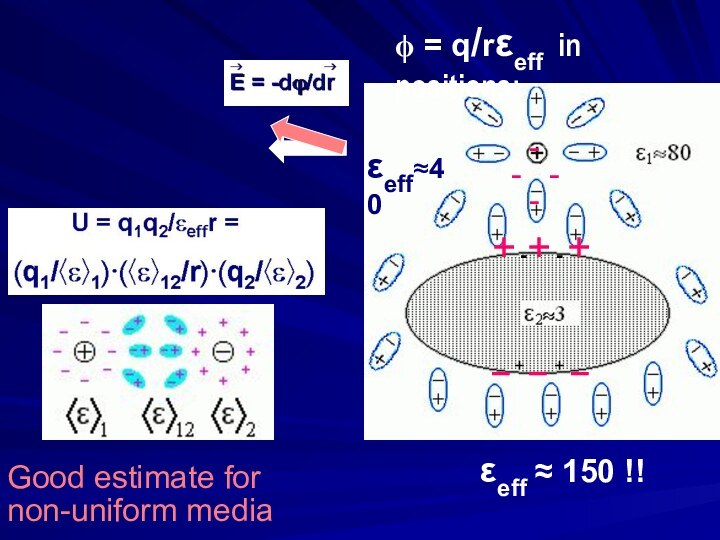
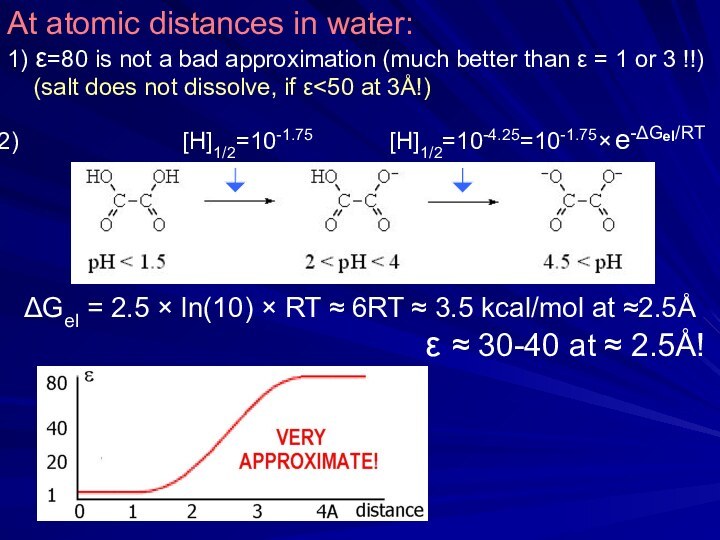
ΔGel = 2.5 × ln(10) × RT ≈ 6RT ≈ 3.5 kcal/mol at ≈2.5Å
+
+
+
-
-
-
ε2
V
ε1
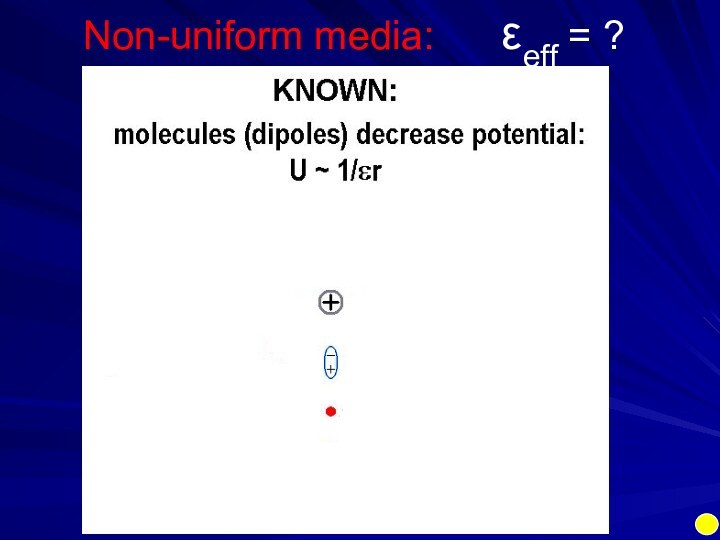
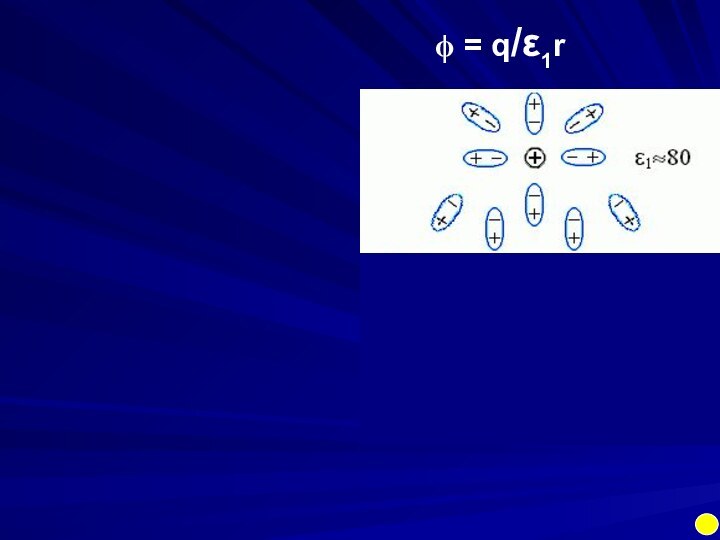
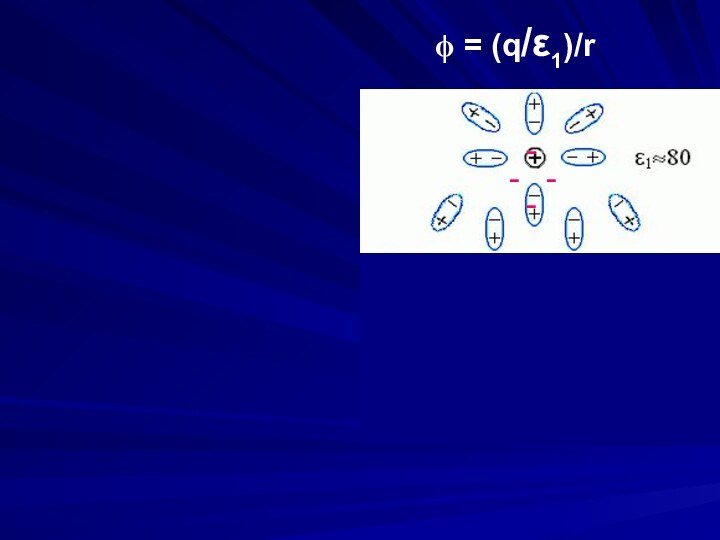
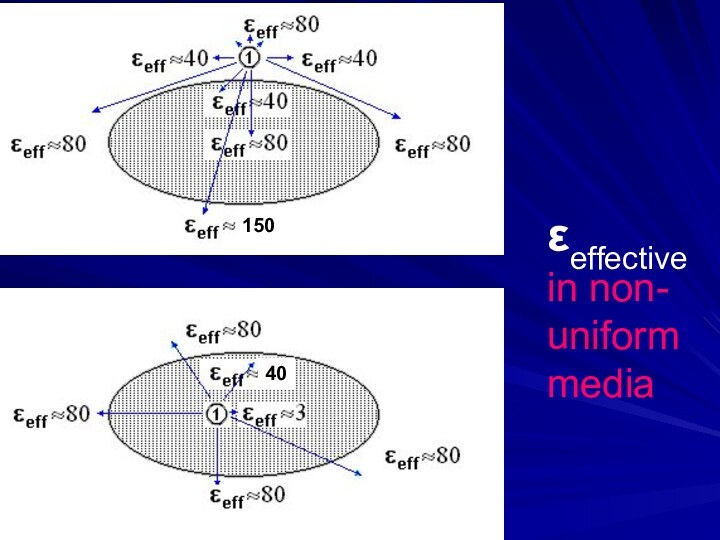
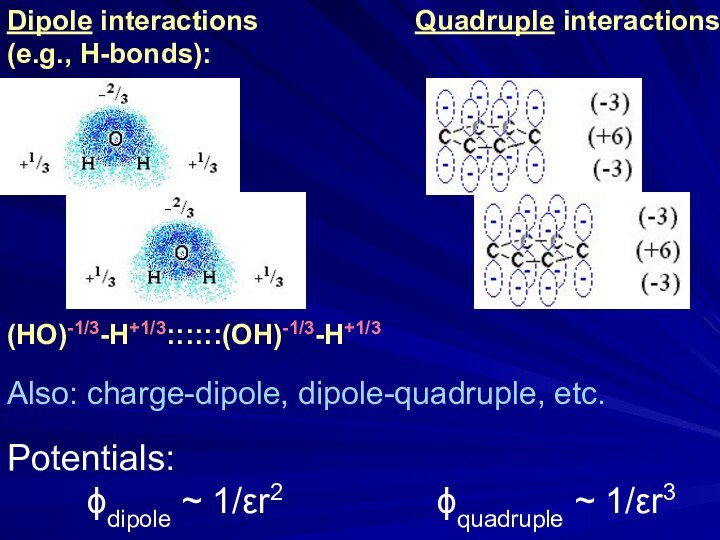
Electrostatics is an example of a multi-body
(charge1, charge2, media, ions) interaction