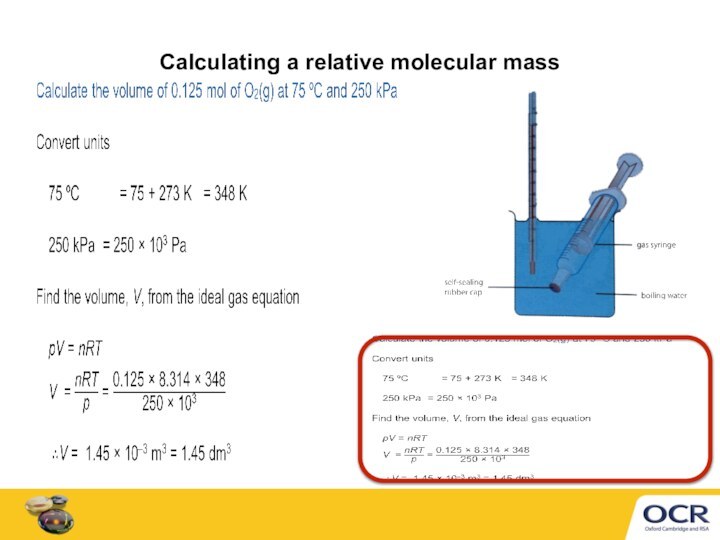
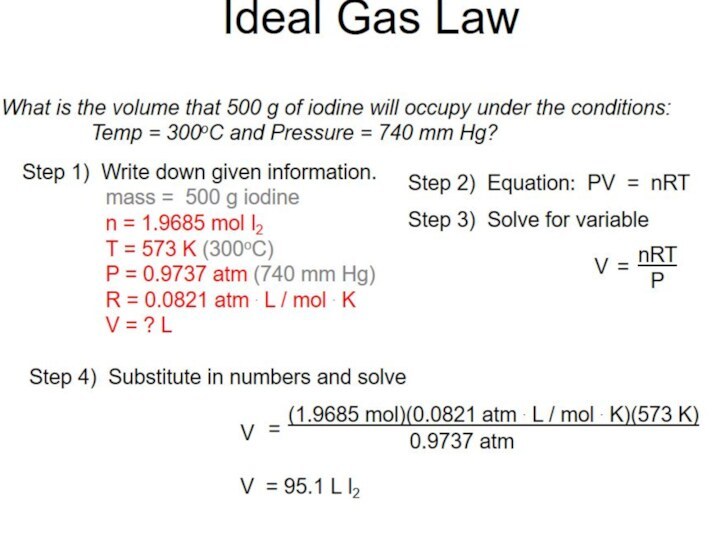
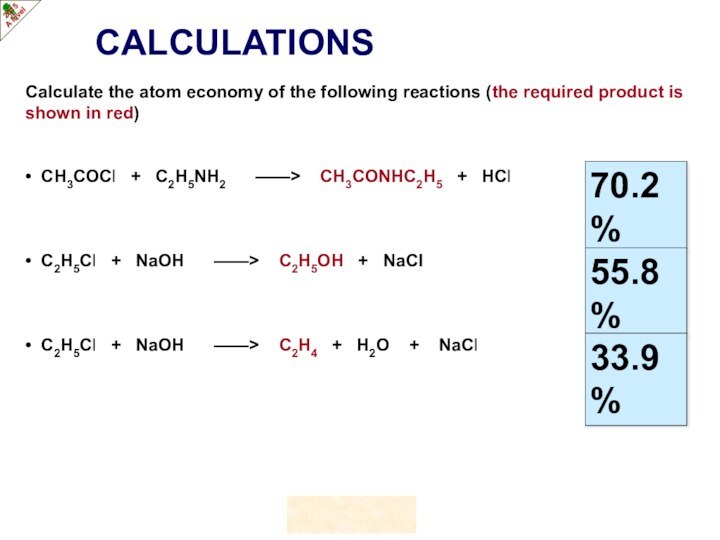
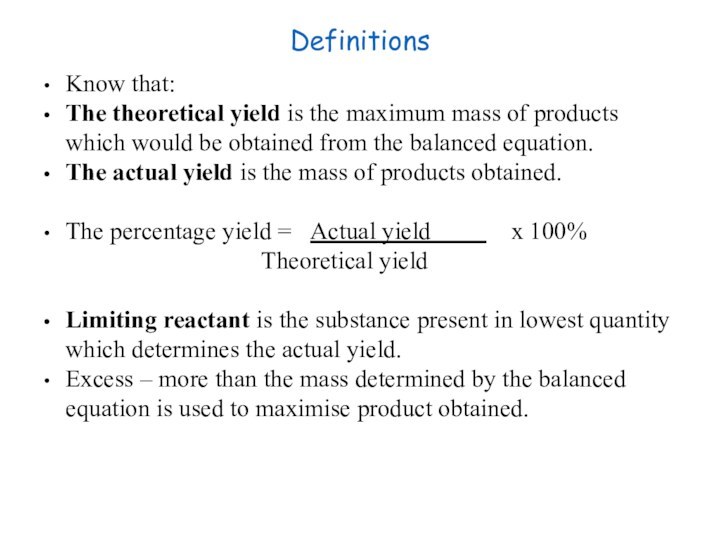
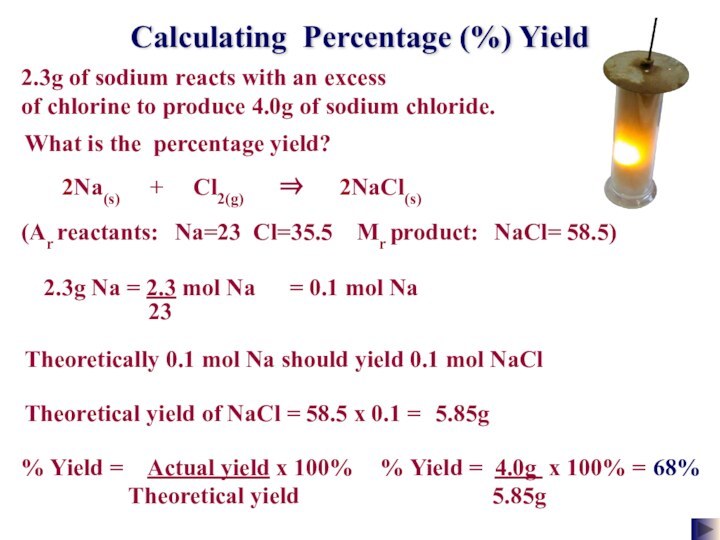
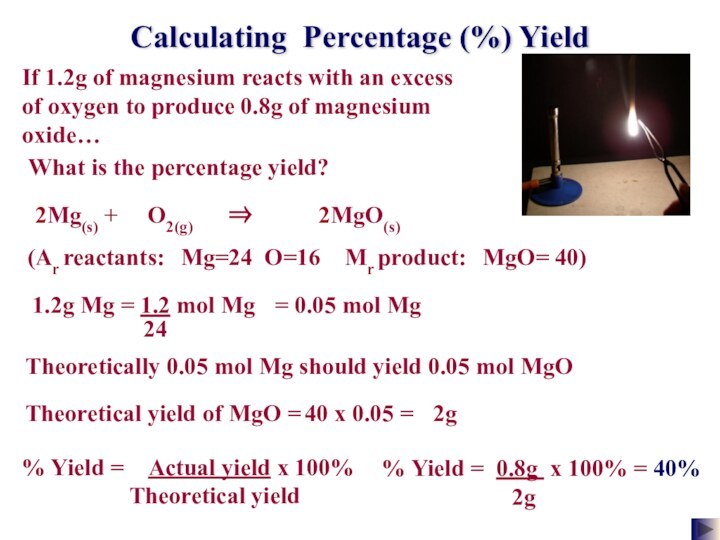
of gas molecules occupies 24.0 dm3
Conditions are not always
room temperature and pressure. A gas volume depends on temperature and pressure.
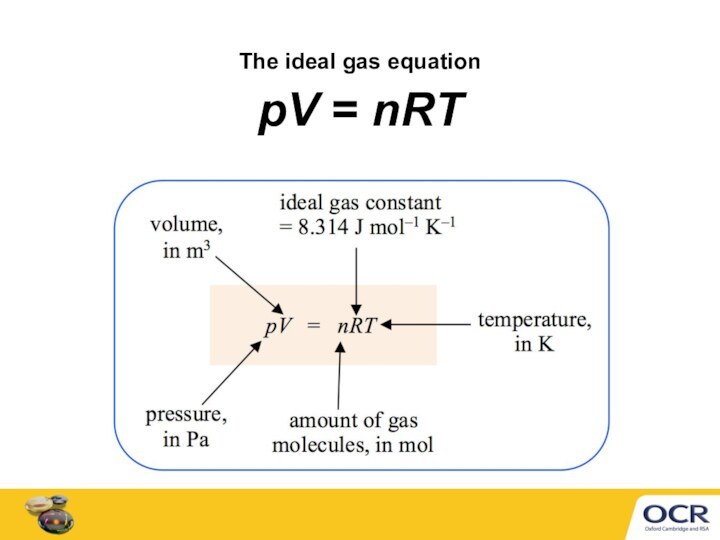
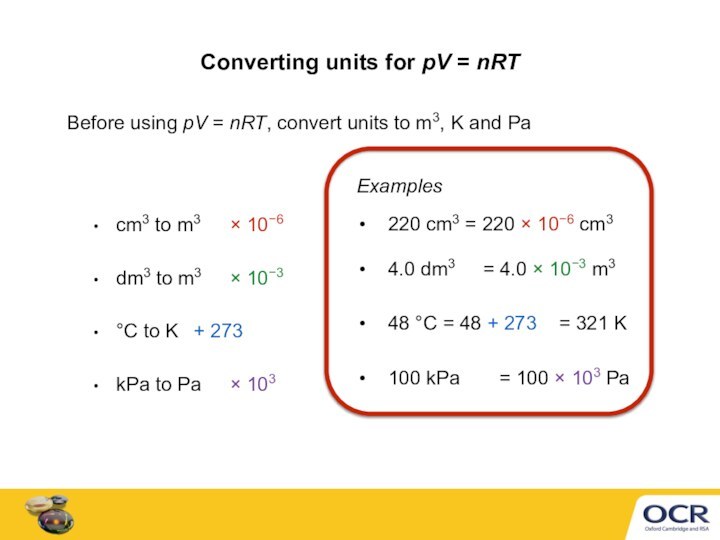
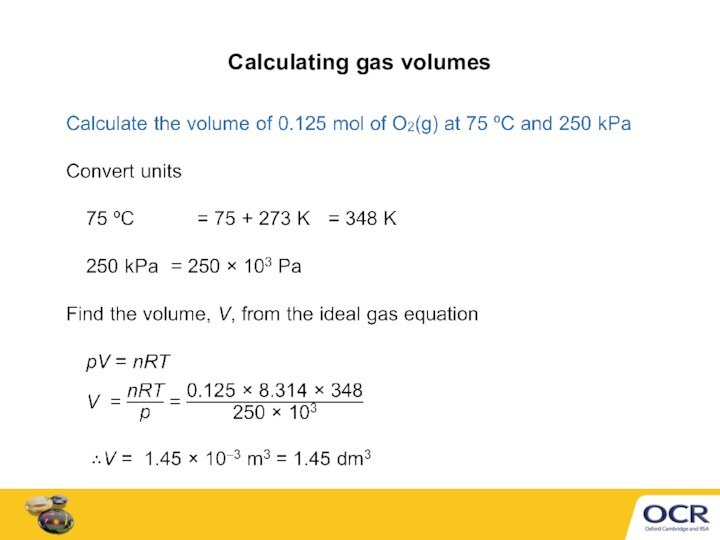
Ideal gas equation can calculate a gas volume, V
at any temperature, T
at any pressure, p