energy (they move).
This means that they move from an
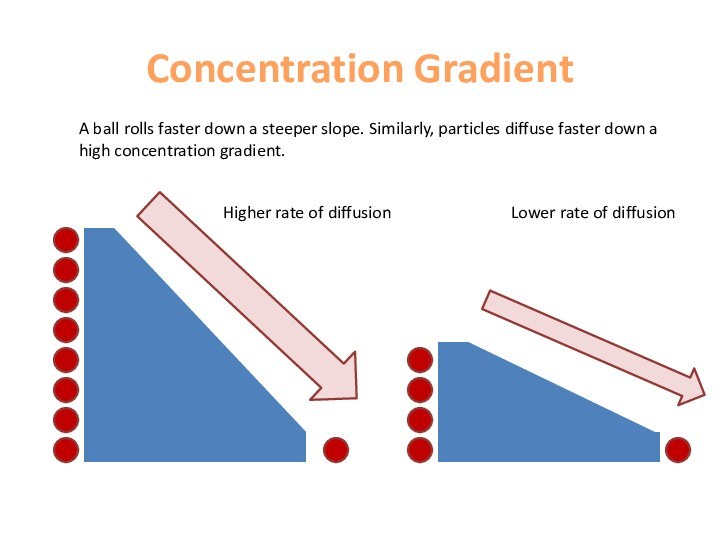
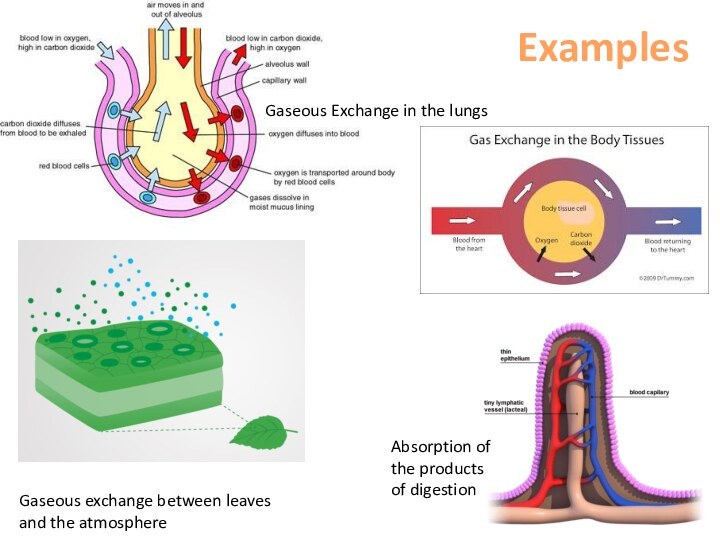
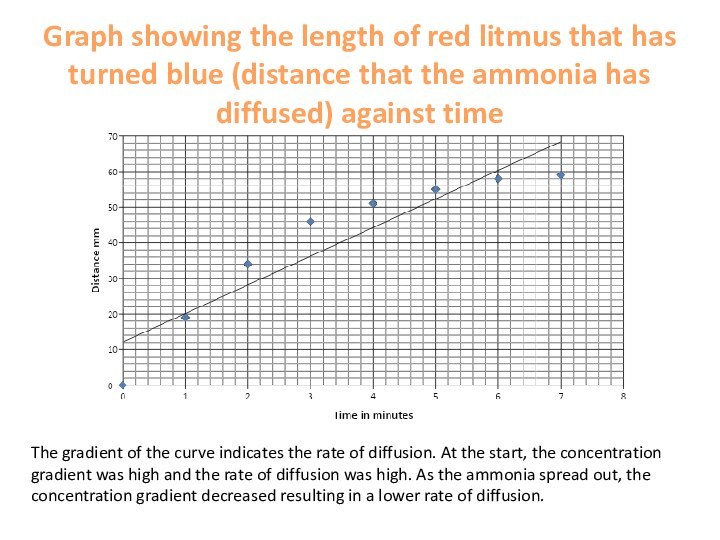
area of high concentration to low concentration.This continues until they are evenly spread out.
The greater the concentration gradient, the greater the rate of diffusion (we ventilate our lungs to maintain a high concentration gradient.
Things diffuse faster in air than in solution.
Raising the temperature increases the rate of diffusion.