Слайд 2
Targeted TB Testing
and Treatment of Latent TB
Infection
Targeted TB testing is used to focus program activities
and provider practices on groups at the highest risk for TB.
Treatment of LTBI substantially reduces the risk that persons infected with M. tuberculosis will progress to TB disease.
Слайд 3
Latent TB Infection (LTBI) diagnosis and treatment
Слайд 4
Latent TB Infection (LTBI)
LTBI is
the presence of M. tuberculosis organisms (tubercle bacilli) without
signs and symptoms or radiographic or bacteriologic evidence of TB disease.
Слайд 5
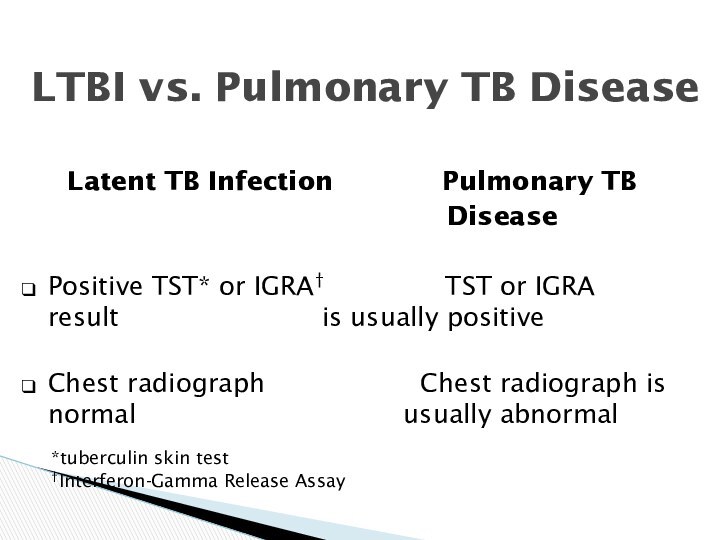
LTBI vs. Pulmonary TB Disease
Latent TB Infection
Pulmonary TB
Disease
Positive TST* or IGRA† TST or IGRA result is usually positive
Chest radiograph Chest radiograph is normal usually abnormal
*tuberculin skin test
†Interferon-Gamma Release Assay
Слайд 6
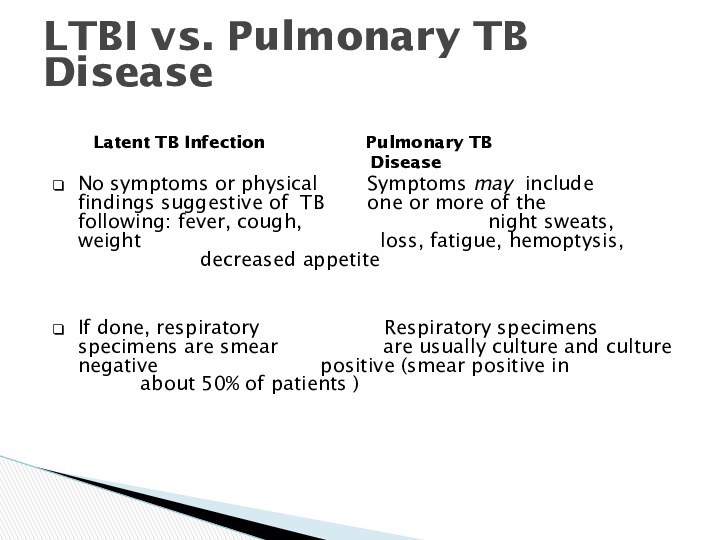
LTBI vs. Pulmonary TB Disease
Latent TB Infection
Pulmonary TB
Disease
No symptoms or physical Symptoms may include findings suggestive of TB one or more of the following: fever, cough, night sweats, weight loss, fatigue, hemoptysis, decreased appetite
If done, respiratory Respiratory specimens specimens are smear are usually culture and culture negative positive (smear positive in about 50% of patients )
Слайд 7
Targeted TB Testing
Essential TB prevention and control strategy
Detects
persons with LTBI who would benefit from treatment
De-emphasizes testing
of groups that are not at high risk for TB
Can help reduce the waste of resources and prevent inappropriate treatment
Слайд 8
Treatment of LTBI – Milestones
Treatment of
persons with LTBI to prevent TB disease is for
more than 3 decades an essential component of TB prevention and control in the United States.
Слайд 9
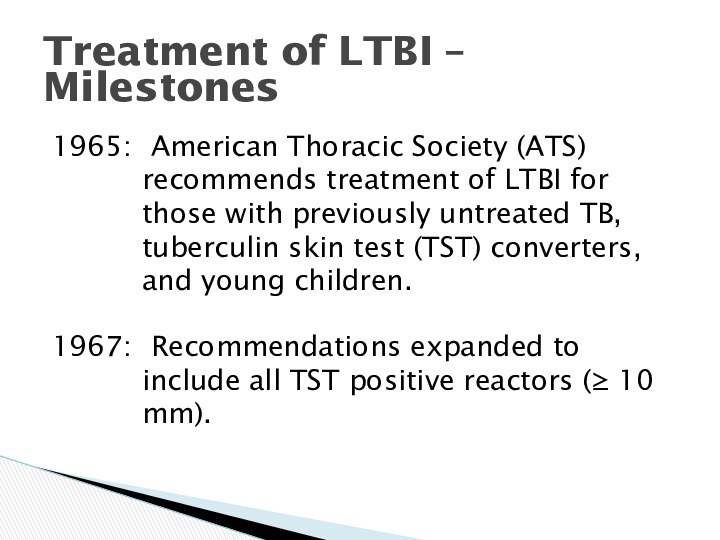
Treatment of LTBI – Milestones
1965: American Thoracic
Society (ATS) recommends treatment of LTBI for those with
previously untreated TB, tuberculin skin test (TST) converters, and young children.
1967: Recommendations expanded to include all TST positive reactors ( 10 mm).
Слайд 10
Treatment of LTBI – Milestones
1974: CDC and ATS
guidelines established for pretreatment screening to decrease risk of
hepatitis associated with treatment
Treatment recommended for persons ≤ 35 years of age
Слайд 11
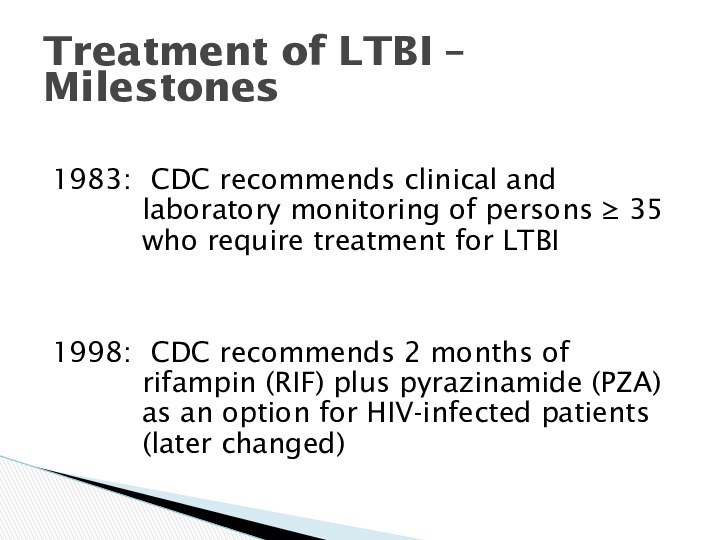
Treatment of LTBI – Milestones
1983: CDC recommends clinical
and laboratory monitoring of persons 35 who require
treatment for LTBI
1998: CDC recommends 2 months of rifampin (RIF) plus pyrazinamide (PZA) as an option for HIV-infected patients (later changed)
Слайд 12
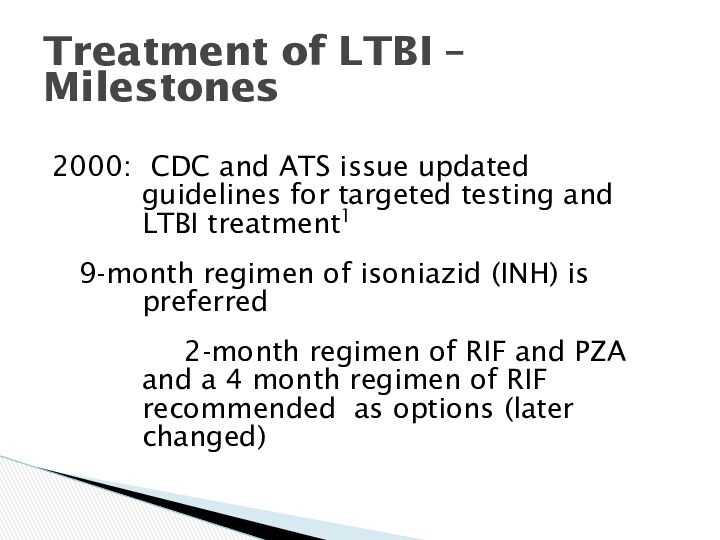
Treatment of LTBI – Milestones
2000: CDC and ATS
issue updated guidelines for targeted testing and LTBI treatment1
9-month regimen of isoniazid (INH) is preferred
2-month regimen of RIF and PZA and a 4 month regimen of RIF recommended as options (later changed)
Слайд 13
Treatment of LTBI – Milestones
2001: Owing to liver injury
and death associated with 2-month regimen of RIF and
PZA, use of this option de-emphasized in favor of other regimens2
2003: 2-month regimen of RIF and PZA generally not recommended — to be used only if the potential benefits outweigh the risk of severe liver injury and death
Слайд 14
Treatment of LTBI – Milestones
2011: CDC recommends 12-doses (3
months) of isoniazid (INH) and rifapentine (RPT) as an
option equal to the standard 9-month INH regimen for certain groups*
Слайд 15
Risk Factors
That Lead to Development of TB Disease
Слайд 16
Persons at Risk for Developing
TB Disease
Persons at high
risk for developing TB disease fall into 2 categories:
Those
who have an increased likelihood of exposure to persons with TB disease
Those with clinical conditions that increase their risk of progressing from LTBI to TB disease
Слайд 17
Increased Likelihood of Exposure to
Persons with TB
Disease
Persons at risk for exposure to persons with TB
disease include:
Close contacts to person with infectious TB
Residents and employees of high-risk congregate settings (e.g., correctional facilities, homeless shelters, health care facilities)
Recent immigrants from TB-endemic regions of the world (within 5 years of arrival to the country)
Слайд 18
Increased Risk for Progression to
TB Disease
Persons more
likely to progress from LTBI to TB disease include:
HIV-infected
persons
Those with a history of prior, untreated TB or fibrotic lesions on chest radiograph
Children 5 years with a positive TST
Слайд 19
Increased Risk for Progression to
TB Disease
Persons more
likely to progress from LTBI to TB disease include:
Underweight
or malnourished persons
Injection drug users
Those receiving TNF-α antagonists for treatment of rheumatoid arthritis or Crohn’s disease
Слайд 20
Increased Risk for Progression to
TB Disease
Persons more likely to progress from LTBI to TB
disease include:
Those with certain medical conditions such as:
Silicosis
Diabetes mellitus
Chronic renal failure or on hemodialysis
Solid organ transplantation (e.g., heart, kidney)
Carcinoma of head or neck
Gastrectomy or jejunoilial bypass
Слайд 21
Testing for M. tuberculosis Infection
Слайд 22
Testing for M. tuberculosis Infection
There are two testing
methods available for the detection of M. tuberculosis infection
Mantoux
tuberculin skin test (TST)
Interferon-gamma release assays (IGRA)
Слайд 23
Mantoux Tuberculin Skin Test
Skin test that produces
delayed-type hypersensitivity reaction in persons with M. tuberculosis infection
TST is useful for:
Determining how many people in a group are infected (e.g., contact investigation)
Examining persons who have symptoms of TB disease
Multiple puncture tests (e.g., Tine Test) are inaccurate and not recommended
Слайд 24
Administering the TST
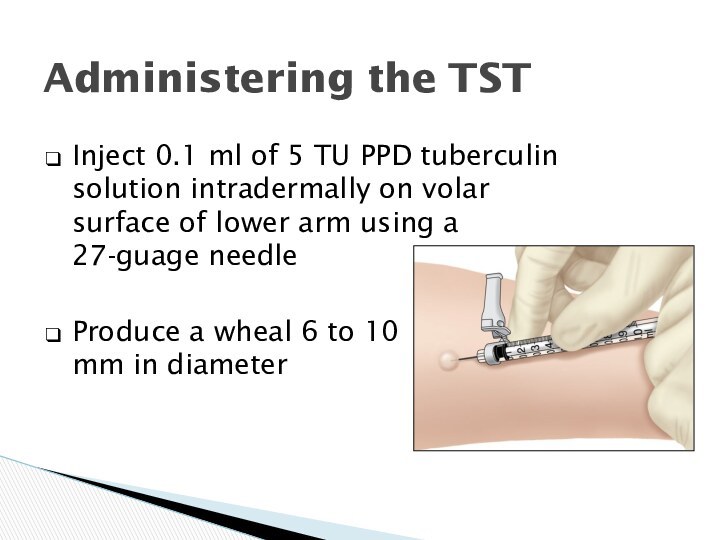
Inject 0.1 ml
of 5 TU PPD tuberculin
solution intradermally on volar surface of lower arm using a 27-guage needle
Produce a wheal 6 to 10 mm in diameter
Слайд 25
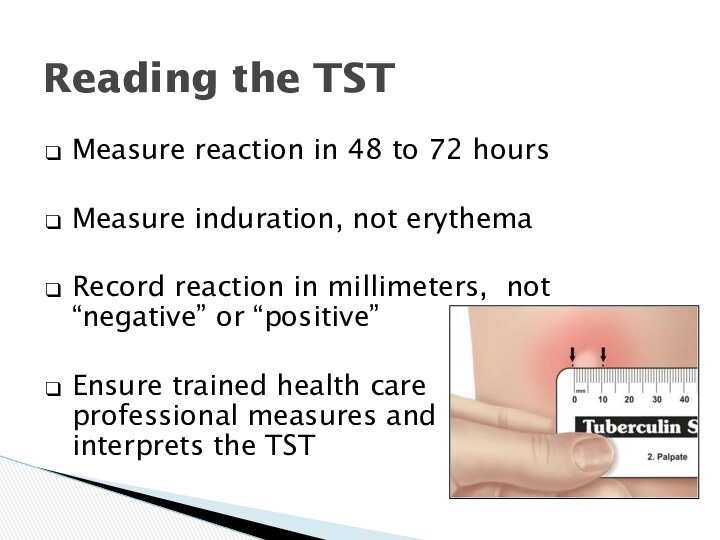
Reading the TST
Measure reaction
in 48 to 72 hours
Measure induration, not erythema
Record reaction
in millimeters, not “negative” or “positive”
Ensure trained health care professional measures and interprets the TST
Слайд 26
Reading the TST
Educate patient
and family regarding significance of a positive TST result
Positive
TST reactions can be measured accurately for up to 7 days
Negative reactions can be read accurately for only 72 hours
Слайд 27
TST Interpretation
5
mm induration is interpreted as positive in
HIV-infected persons
Close contacts
to an infectious TB case
Persons with chest radiographs consistent with prior untreated TB
Слайд 28
TST Interpretation
5 mm induration is
interpreted as positive in
Organ transplant recipients
Other immunosuppressed patients (e.g.
, those taking the equivalent of > 15 mg/d of prednisone for 1 month or those taking TNF-α antagonists)
Слайд 29
TST Interpretation
10
mm induration is interpreted as positive in
Recent immigrants
Injection
drug users
Residents or employees of congregate settings
Mycobacteriology laboratory personnel
Слайд 30
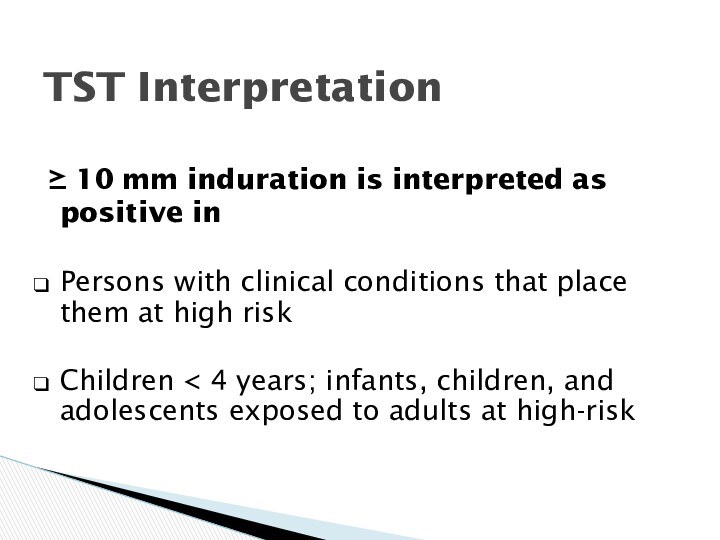
TST Interpretation
10 mm
induration is interpreted as positive in
Persons with clinical
conditions that place them at high risk
Children < 4 years; infants, children, and adolescents exposed to adults at high-risk
Слайд 31
TST Interpretation
15
mm induration is interpreted as positive in
Persons with no
known risk factors for TB.
Although skin testing programs should be conducted only among high-risk groups, certain individuals may require TST for employment or school attendance. Diagnosis and treatment of LTBI should always be tied to risk assessment.
Слайд 32
Factors That May Cause False-
Positive TST Reactions
Nontuberculous myobacteria
Reactions caused by nontuberculous mycobacteria are usually 10
mm of induration
BCG vaccination
Reactivity in BCG vaccine recipients generally wanes over time;
positive TST result is likely due to TB infection if risk factors are present
Слайд 33
Factors That May Cause False-
Negative TST Reactions
Anergy
Inability to react to a TST because of a
weakened immune system
Usefulness of anergy testing in TST-negative persons who are HIV infected has not been demonstrated
Слайд 34
Factors That May Cause False-
Negative TST Reactions
Recent TB Infection
Defined as less than 10 weeks after
exposure
Very young age
Newborns (< 6 months)
Слайд 35
Factors That May Cause False-
Negative TST Reactions
Live virus vaccination
For example, measles or smallpox
Can temporarily suppress
TST reactivity
Overwhelming TB Disease
Poor TST administration technique
For example, TST injection too shallow or too deep, or wheal is too small
Слайд 36
Boosting
Some people with LTBI may have a negative
skin test reaction when tested years after infection because
of a waning response.
An initial skin test may stimulate (boost) the ability to react to tuberculin.
Positive reactions to subsequent tests may be misinterpreted as new infections rather than “boosted” reactions.
Слайд 37
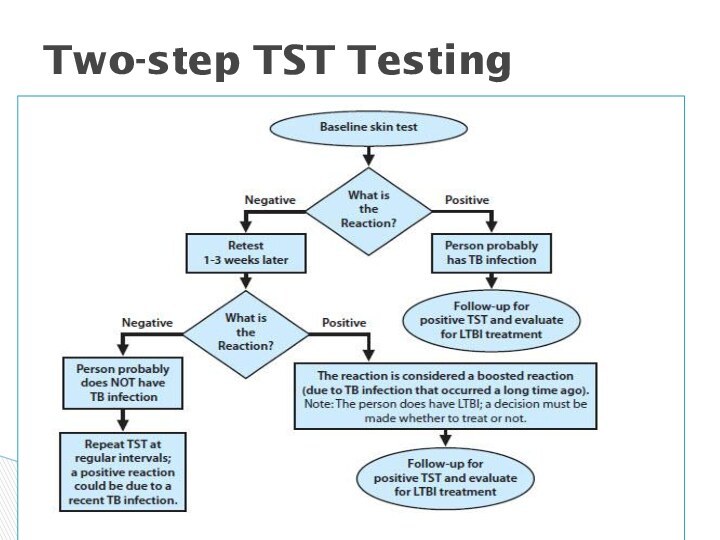
Two-Step Testing
A strategy to determine the difference
between boosted reactions and reactions due to recent infection.
If
1st test positive, consider infected; if negative, give 2nd test 1–3 weeks later
If 2nd test positive, consider infected; if negative, consider uninfected
Use two-step tests for initial baseline skin testing of adults who will be retested periodically (e.g., health care workers).
Слайд 39
Interferon-Gamma Release Assays (IGRAs)
Слайд 40
Interferon-Gamma Release
Assays (IGRAs)
Whole-blood test used to detect
M. tuberculosis infection
Two U.S. Food and Drug Administration (FDA)
approved IGRAs are commercially available in the U.S.
QuantiFERON® -TB Gold-in-tube test (QFT-GIT)
T.SPOT® .TB test (T-Spot)
Слайд 41
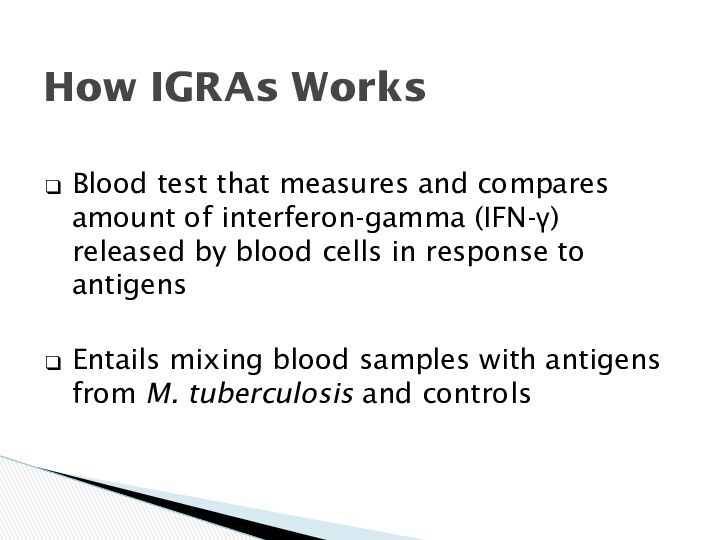
How IGRAs Works
Blood test that measures and
compares amount of interferon-gamma (IFN-) released by blood cells
in response to antigens
Entails mixing blood samples with antigens from M. tuberculosis and controls
Слайд 42
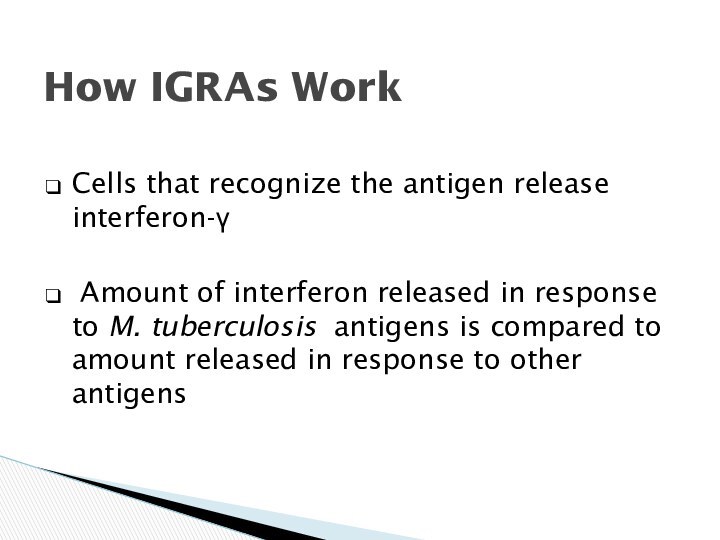
How IGRAs Work
Cells that recognize the antigen release
interferon-
Amount of interferon released in response to M.
tuberculosis antigens is compared to amount released in response to other antigens
Слайд 43
Administering IGRAs
Confirm and arrange for delivery of blood
sample within specific time-frame to ensure viability of blood
samples
Draw blood sample according to test manufacturer’s instructions
Schedule a follow up appointment to receive test results, medical evaluation and possible treatment if needed
Слайд 44
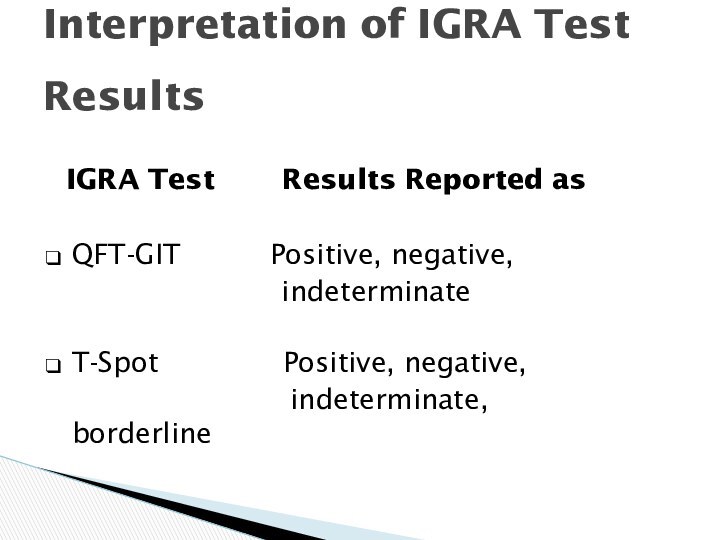
Interpretation of IGRA Test
Results
IGRA
Test Results Reported as
QFT-GIT
Positive, negative,
indeterminate
T-Spot Positive, negative,
indeterminate, borderline
Слайд 45
Advantages of IGRAs
Requires a single patient visit
to conduct test
Results can be available within 24 hours
Does
not boost responses measured by subsequent tests
Prior BCG vaccination does not cause false-positive IGRA test result
Слайд 46
Disadvantages/Limitations of
IGRAs
Errors in collecting and transporting
blood, or in interpreting assays can decrease accuracy of
IGRAs
Limited data on use of IGRAs to predict who will progress to TB disease in the future
Слайд 47
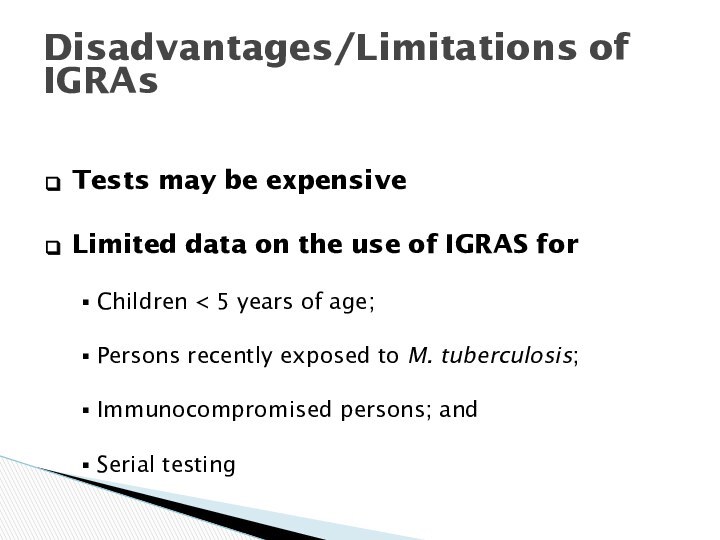
Disadvantages/Limitations of IGRAs
Tests may be expensive
Limited data
on the use of IGRAS for
Children < 5 years
of age;
Persons recently exposed to M. tuberculosis;
Immunocompromised persons; and
Serial testing
Слайд 48
Selecting a Test to Detect TB
Infection
IGRAs are preferred method of testing for
Groups of people
who have poor rates of returning to have TST read
Persons who have received BCG vaccine
TST is the preferred method of testing for
Children under the age of 5
Слайд 49
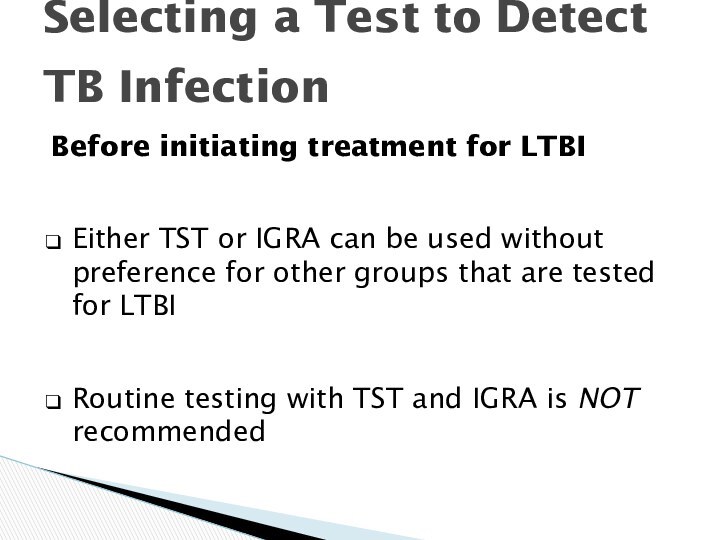
Selecting a Test to Detect
TB Infection
Before
initiating treatment for LTBI
Either TST or IGRA can be
used without preference for other groups that are tested for LTBI
Routine testing with TST and IGRA is NOT recommended
Слайд 50
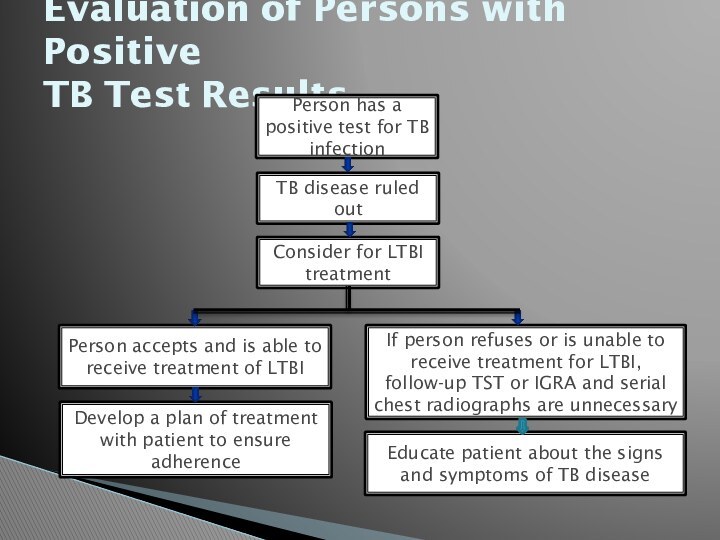
Evaluation of Persons with Positive
TB Test Results
Слайд 52
Initiating Treatment
Before initiating treatment for LTBI
Rule out TB
disease by history, physical examination, chest radiography and, when
indicated, bacteriologic studies
Determine prior history of treatment for LTBI or TB disease
Assess risks and benefits of treatment
Determine current and previous drug therapy
Слайд 53
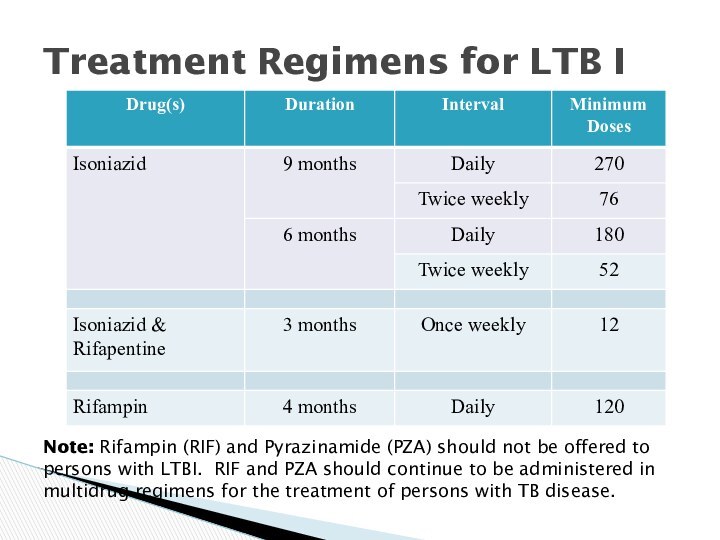
Treatment Regimens for LTB I
Note: Rifampin (RIF)
and Pyrazinamide (PZA) should not be offered to persons
with LTBI. RIF and PZA should continue to be administered in multidrug regimens for the treatment of persons with TB disease.
Слайд 54
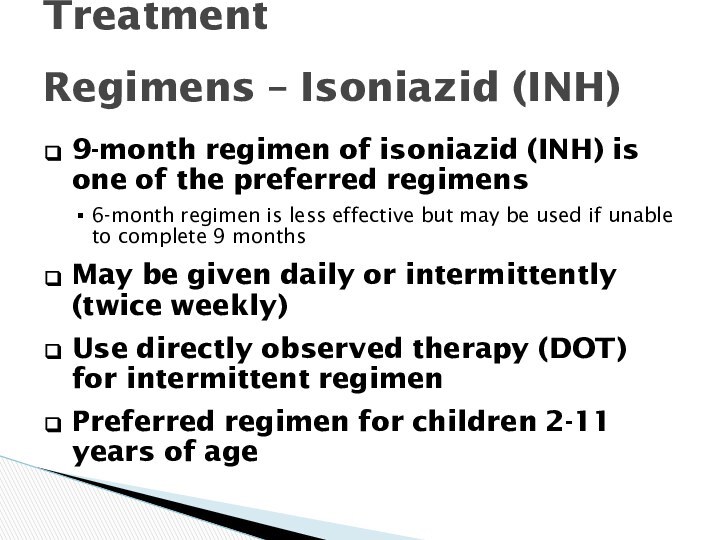
Latent TB Infection Treatment
Regimens – Isoniazid (INH)
9-month regimen of isoniazid (INH) is one of the
preferred regimens
6-month regimen is less effective but may be used if unable to complete 9 months
May be given daily or intermittently (twice weekly)
Use directly observed therapy (DOT) for intermittent regimen
Preferred regimen for children 2-11 years of age
Слайд 55
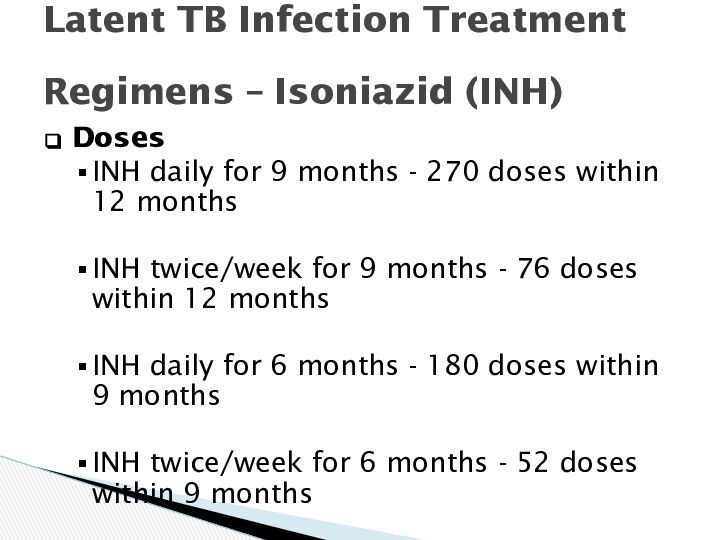
Latent TB Infection Treatment
Regimens – Isoniazid (INH)
Doses
INH
daily for 9 months - 270 doses within 12
months
INH twice/week for 9 months - 76 doses within 12 months
INH daily for 6 months - 180 doses within 9 months
INH twice/week for 6 months - 52 doses within 9 months
Слайд 56
LTBI Treatment Regimens – Isoniazid (INH) and Rifapentine
(RPT)
3-month regimen of INH and RPT is an
option equal to 9-month INH regimen for treating LTBI in certain groups, such as otherwise healthy people, 12 years of age and older, who were recently in contact with infectious TB or who had tuberculin skin test conversions or positive blood test for TB
Must use directly observed therapy (DOT)
Слайд 57
LTBI Treatment Regimens – Isoniazid (INH) and Rifapentine
(RPT)
Not recommended for children younger than 12 years
of age, HIV-infected people taking antiretroviral therapy, pregnant women, or women expecting to be pregnant within the 12-week regimen
INH and RPT once a week for 3 months - 12 doses within 4 months
Слайд 58
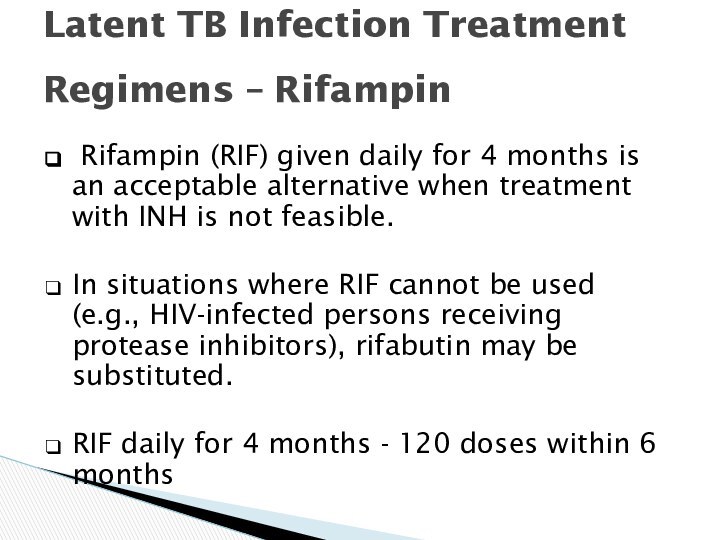
Latent TB Infection Treatment
Regimens – Rifampin
Rifampin
(RIF) given daily for 4 months is an acceptable
alternative when treatment with INH is not feasible.
In situations where RIF cannot be used
(e.g., HIV-infected persons receiving protease inhibitors), rifabutin may be substituted.
RIF daily for 4 months - 120 doses within 6 months
Слайд 59
LTBI Treatment Regimens for Specific
Situations – HIV-Infected
Persons
HIV-Infected Persons
Consult an expert in managing HIV and TB
INH
daily for 9-mo, rather than 6-mo, is optimal: 270 doses within 12 months
RIF is generally contraindicated for persons taking protease inhibitors or delavirdine
Rifabutin with dose adjustments can sometimes be substituted for RIF
INH/RPT regimen not recommended for HIV-infected people taking antiretroviral therapy
Слайд 60
LTBI Regimens for Specific
Situations – Fibrotic Lesions
Persons
with Fibrotic Lesions Suggesting Previous TB
Should be treated for
LTBI if they have
A positive TST reaction (at least 5 mm) or IGRA result
No symptoms of infectious TB disease
No history of treatment for TB disease
Treat only after active disease excluded with sputum testing
Acceptable regimens include
9 months of INH
4 months of RIF (with or without INH)
3 months of INH and RPT (12-dose regimen)
Слайд 61
LTBI Treatment Regimens for Specific Situations – Multidrug-Resistant
TB
Contacts of Persons with Multidrug-Resistant TB
Consider risk for progressing
to MDR disease before recommending LTBI treatment
When prescribing treatment for these contacts, consult an MDR TB expert
Слайд 62
LTBI Treatment Regimens for
Specific Situations - Pregnancy
Pregnancy
and Breast-Feeding
9 months of INH daily or twice weekly;
give with vitamin B6
If cannot take INH, consult with TB expert
Women at high risk for progression to TB disease should not delay LTBI treatment; monitor carefully
Breast-feeding not contraindicated
Слайд 63
Completion of Therapy
Completion of therapy is based on
the total number of doses administered, not on duration
alone.
Слайд 64
Management of Patient Who
Missed Doses
Extend or re-start
treatment if interruptions were frequent or prolonged enough to
preclude completion
When treatment has been interrupted for more than 2 months, patient should be examined to rule out TB disease
Recommend and arrange for DOT as needed
Слайд 66
Clinical Monitoring
Instruct patient to report signs and
symptoms of adverse drug reactions:
Fever
Headache
Rash
Anorexia, nausea, vomiting, or
abdominal pain in right upper quadrant
Fatigue or weakness
Dark urine
Persistent numbness in hands or feet
Слайд 67
Clinical Monitoring
Monthly visits should include a brief
physical exam and a review of:
Rationale for treatment
Adherence with
therapy
Symptoms of adverse drug reactions
Plans to continue treatment
Слайд 68
Clinical Monitoring
Incidence of hepatitis in persons taking INH
is lower than previously thought (as low as 0.1%)
Hepatitis
risk increases with age
Uncommon in persons < 20 years old
Nearly 2% in persons 50 to 64 years old
Risk increases with underlying liver disease or heavy alcohol consumption
Слайд 69
Laboratory Monitoring
Baseline liver function tests (e.g., AST,
ALT, and bilirubin) are not necessary except for patients
with risk factors:
HIV infection
History of liver disease
Regular alcohol use
Pregnancy or in early postpartum period
Слайд 70
Laboratory Monitoring
Repeat laboratory monitoring if patient has:
Abnormal
baseline results
Current or recent pregnancy
High risk for adverse reactions
Symptoms
of adverse reaction
Liver enlargement or tenderness during examination
Слайд 71
Laboratory Monitoring
Asymptomatic elevation of hepatic enzymes seen
in 10%-20% of people taking INH
Levels usually return to
normal after completion of therapy
Discontinue treatment if transminase level exceeds 3 times the upper limit of normal if patient has symptoms of hepatoxicity, and 5 times the upper limit of normal if patient is asymptomatic