- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Phytoremediation of heavy metals-concepts and applications
Содержание
- 2. Uses of phytoremediation air soils, sediments
- 3. Uses of phytoremediation (cont.) inorganics:- metals
- 4. Uses of phytoremediation (cont.) farming polluted
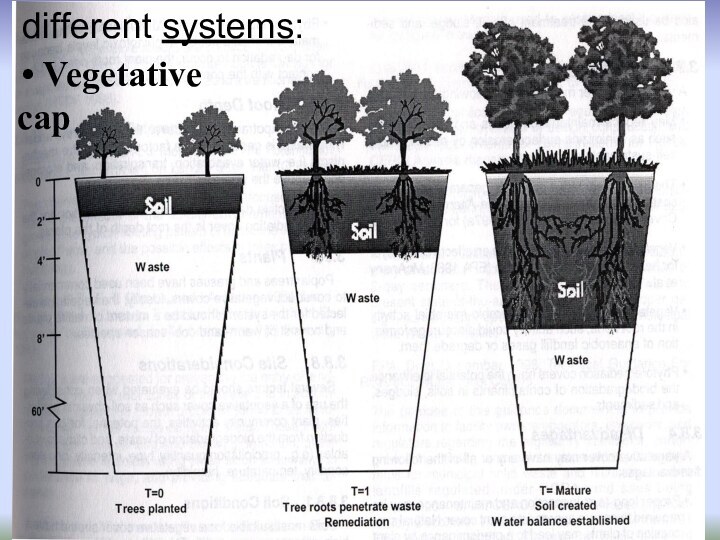
- 5. Hydraulic barrierdifferent systems:
- 6. Vegetative capdifferent systems:
- 7. Constructed wetlandsdifferent systems:
- 8. different systems: hydroponics with polluted wastewater
- 9. Roots of mustardExtend into effluentActing as filters for heavy metals
- 10. Uses of phytoremediation (cont.) high tolerance
- 11. Uses of phytoremediation (cont.) treesPopular plants for phytoremediationvarious organicsmetalspoplarwillowgum treeyellow poplar
- 12. Uses of phytoremediation (cont.) For inorganicsPopular plants for phytoremediation grasses (cont.):Brassica junceaAlyssumThlaspiBrassicaceae:
- 13. Uses of phytoremediation (cont.)Popular plants for phytoremediation(cont.):hempkenafbamboovarious grasses red fescuebuffalo grassfor organicsfor inorganics
- 14. Uses of phytoremediation (cont.)Popular plants for phytoremediationparrot feather poplar, willow spartinahalophytessalicorniareedaquatic plantscattailfor organicsfor inorganics
- 15. Advantages & Limitations of Phytoremediation
- 16. PhytoremediationMechanical/chemical treatment Soil washing Excavation + reburial Chemical cleanup of soil/water Combustion
- 17. Phytoremediation vs. Mechanical/chemical treatment CheaperAdvantages of phytoremediation~10 - 100xExcavation & reburial: up to $1 million/acreRevegetation: ~$20,000/acre
- 18. Phytoremediation vs. Mechanical/chemical treatmentAdvantages of phytoremediation (cont.)
- 19. Limitations of phytoremediationPhytoremediation vs. Mechanical/chemical treatment (cont.)
- 20. Limitations of phytoremediation (cont.)Phytoremediation vs. Mechanical/chemical treatment
- 21. Limitations of phytoremediation (cont.)Phytoremediation vs. Mechanical/chemical treatment
- 22. So, when choose phytoremediation? Sufficient time available
- 23. Techniques/strategies of phytoremediation phytoextraction (or phytoaccumulation), phytostabilization, Phytostimulation,phytofiltration, phytovolatilization, and phytodegradation
- 24. PhytoextractionPhytoextraction (also known as phytoaccumulation, phytoabsorption or
- 25. accumulationphytoextractionPhytoremediation processes
- 26. Phytoextraction: pollutant accumulated in harvestable plant tissues
- 27. Phytostabilization Phytostabilization or phytoimmobilization is the use
- 28. Phytoremediation processes
- 29. Phytoremediation processesphytostabilization
- 30. Phytostabilization: pollutant immobilized in soil
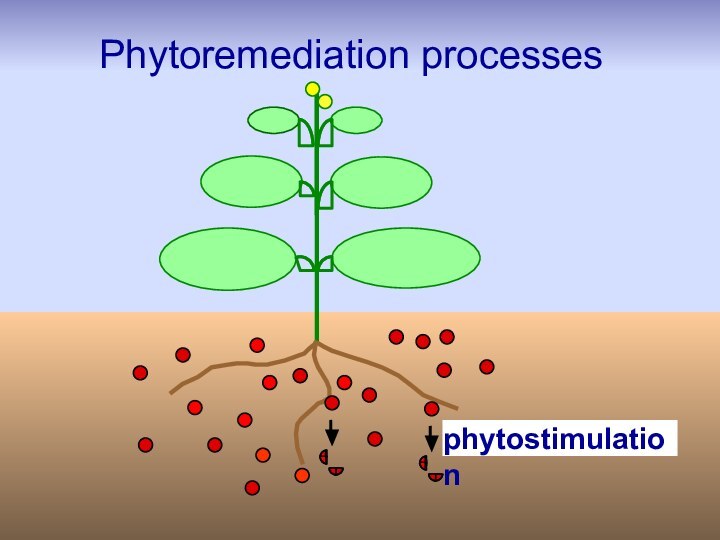
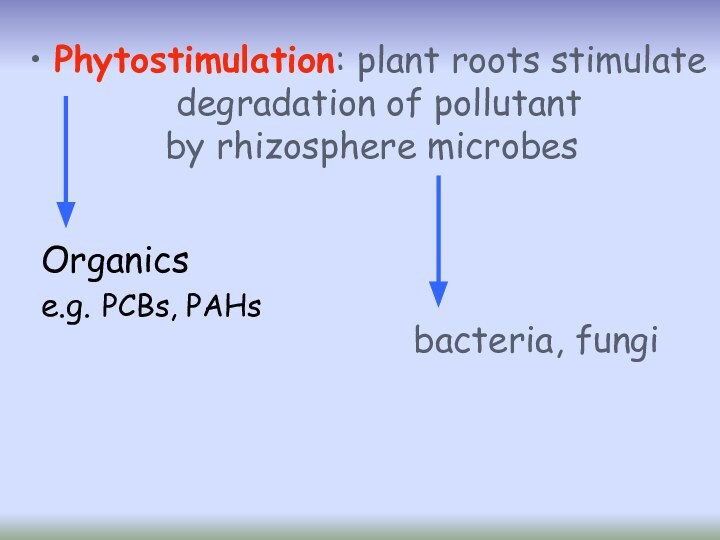
- 31. phytostimulationPhytoremediation processes
- 32. Phytostimulation: plant roots stimulate degradation of pollutant by rhizosphere microbes
- 33. Phytodegradation Phytodegradation is the degradation of organic
- 34. phytodegradationPhytoremediation processes
- 35. Phytodegradation: plants degrade pollutant, with/without uptake, translocationCertain organicse.g. TCE, TNT, atrazine
- 36. Phytovolatilization Phytovolatilization is the uptake of pollutants
- 37. Phytoremediation processesphytovolatilization
- 38. Phytovolatilization: pollutant released in volatile form into the air
- 39. RhizodegradationRhizodegradation refers to the breakdown of organic
- 40. Rhizofiltrationwater
- 41. Rhizofiltration: pollutant removed from water by plant roots in hydroponic systemmetalsmetalloidsradionuclides
- 42. Phytofiltration Phytofiltration is the removal of pollutants
- 43. Rhizofiltration Hydroponics for metal remediation:75% of metals removed from mine drainageInvolves: phytoextraction phytostabilization
- 44. Constructed wetland for Se remediation:Involves: phytoextraction
- 45. Phytodesalination Phytodesalination refers to the use of
- 46. stabilizationdegradationvolatilizationaccumulationPhytoremediation applications may involve multiple processes at once
- 47. Summary of phytoremediation techniques
- 48. Natural attenuation: polluted site left alone
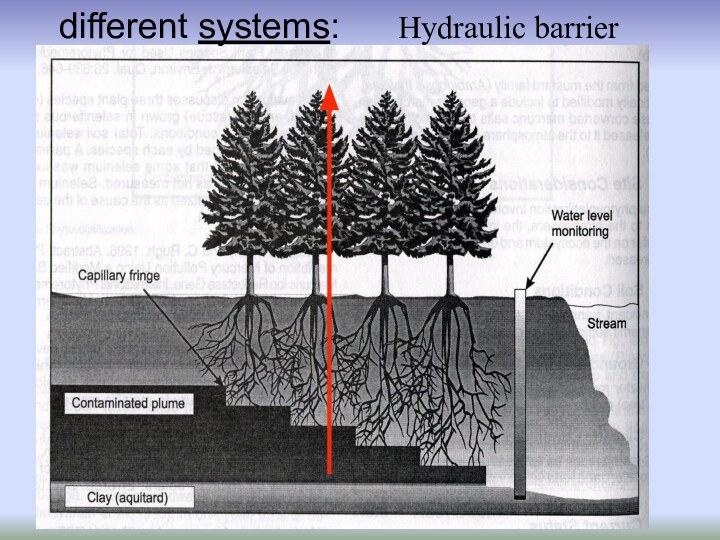
- 49. Hydraulic barrierWater flow redirectedPollutants intercepted
- 50. Heavy metals problems in the context of PHYTOREMEDIATION
- 51. heavy metals originate from extraction of ores
- 52. Anthropogenic sourcesSources of heavy metals in the
- 53. Sources of HM
- 54. Harmful effects of heavy metals on human
- 55. Harmful effects of HM
- 56. Cleanup of heavy metal-contaminated soilsCleanup of heavy
- 57. Phytoremediation – a green solution to the
- 58. Purpose of phytoremediationrisk containment (phytostabilization); phytoextraction of
- 59. Phytoextraction of heavy metalsThe main and
- 60. Phytoextraction: two key factors The phytoextraction potential
- 61. Bioavailability of HM in soilsChemical composition and
- 62. Phytoextraction: two modesNatural conditions: no soil amendm.Induced
- 63. MetallophytesMetallophytes are plants that are specifically adapted
- 64. Hyperaccumulation in plantsThe following concentration criteria for
- 65. HyperaccumulatorsThe most commonly postulated hypothesis regarding the
- 66. Quantification of phytoextraction efficiencyBioconcentration factor indicates the
- 67. Quantification of phytoextraction efficiencyAccumulation factor (A) can
- 68. Fate of plants used for phytoextraction
- 69. PhytominingAdvantages:- can be combusted to get energy
- 70. Use of constructed wetlands for phytoremediationConstructed wetlands
- 71. Mechanism of heavy metals’ uptake, translocation, and
- 72. Role of phytochelatins and metallothioneins in phytoextraction
- 73. Limitations of phytoremediationLong time required Hyperaccumulators are
- 74. Future trends in phytoremediationPhytoremediation is a relatively
- 75. Future challenges in phytoremediationPhytoremediation efficiency of different
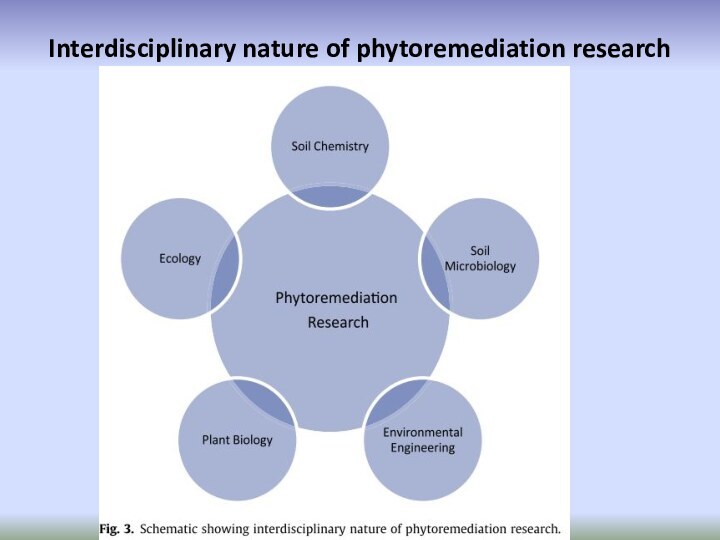
- 76. Interdisciplinary nature of phytoremediation research
- 77. ConclusionsPhysical and chemical methods for clean-up and
- 78. Скачать презентацию
- 79. Похожие презентации




































Слайд 3
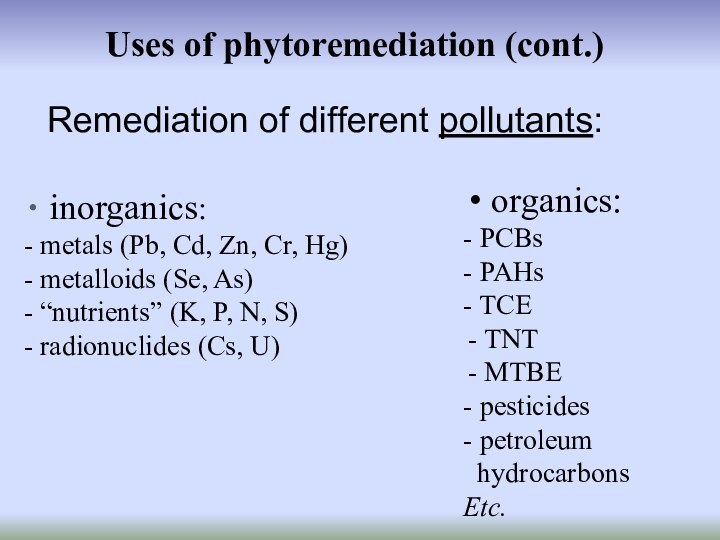
Uses of phytoremediation (cont.)
inorganics:
- metals (Pb,
Cd, Zn, Cr, Hg)
- metalloids (Se, As)
- “nutrients” (K,
P, N, S)- radionuclides (Cs, U)
Remediation of different pollutants:
organics:
- PCBs
- PAHs
- TCE
TNT
MTBE
- pesticides
- petroleum
hydrocarbons
Etc.
Слайд 4
Uses of phytoremediation (cont.)
farming polluted soil
irrigation with polluted groundwater
letting trees tap into groundwater
letting plants filter water streamsconstructed wetlands, hydroponics
Remediation using different systems:
Слайд 10
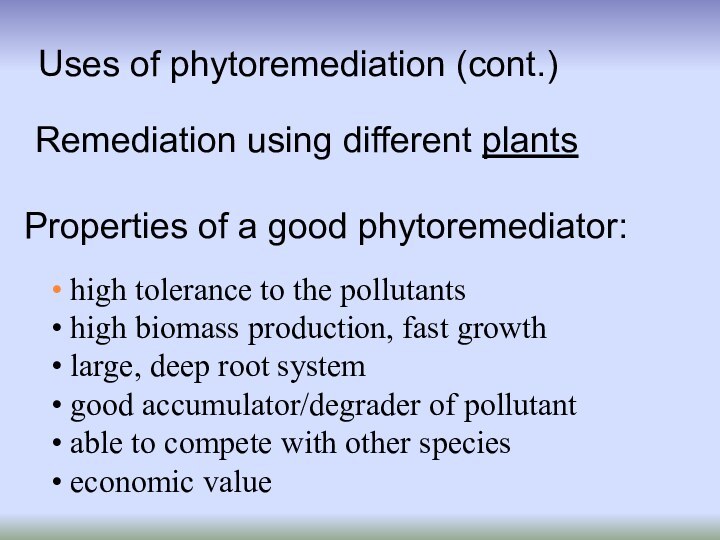
Uses of phytoremediation (cont.)
high tolerance to
the pollutants
high biomass production, fast growth
large,
deep root systemgood accumulator/degrader of pollutant
able to compete with other species
economic value
Properties of a good phytoremediator:
Remediation using different plants
Слайд 11
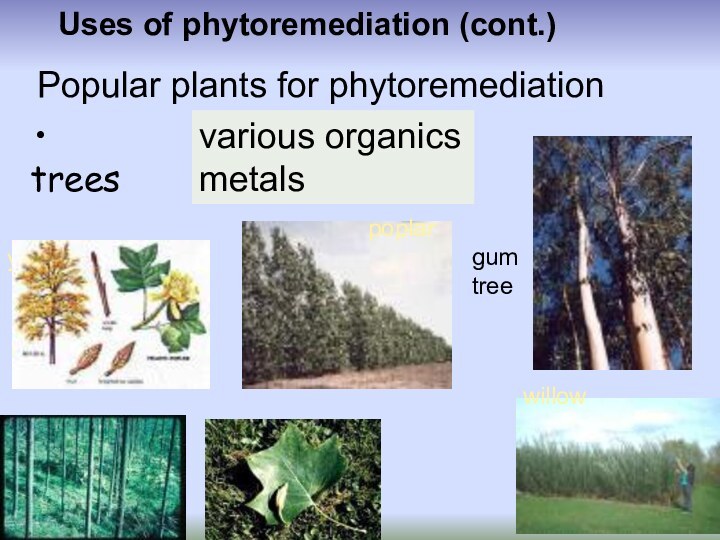
Uses of phytoremediation (cont.)
trees
Popular plants for
phytoremediation
various organics
metals
poplar
willow
gum tree
yellow poplar
Слайд 12
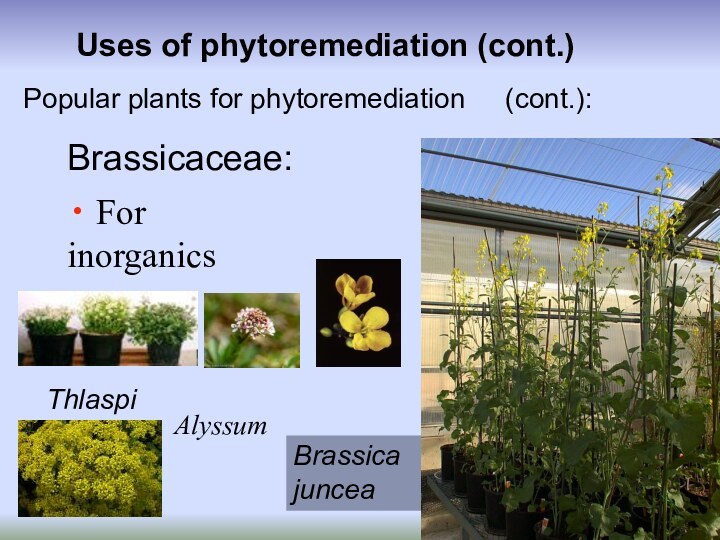
Uses of phytoremediation (cont.)
For inorganics
Popular plants
for phytoremediation
grasses
(cont.):
Brassica juncea
Alyssum
Thlaspi
Brassicaceae:
Слайд 13
Uses of phytoremediation (cont.)
Popular plants for phytoremediation
(cont.):
hemp
kenaf
bamboo
various
grasses
red fescue
buffalo grass
for organics
for inorganics
Слайд 14
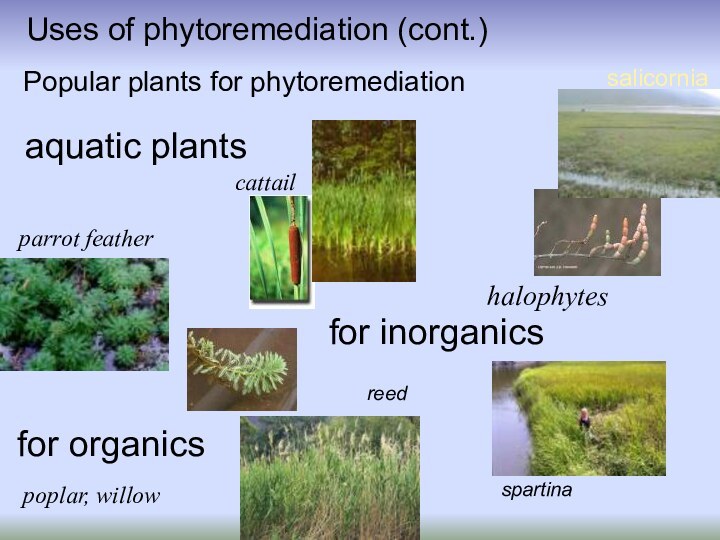
Uses of phytoremediation (cont.)
Popular plants for phytoremediation
parrot
feather
poplar, willow
spartina
halophytes
salicornia
reed
aquatic plants
cattail
for organics
for inorganics
Слайд 16
Phytoremediation
Mechanical/chemical treatment
Soil washing
Excavation + reburial
Chemical
cleanup of soil/water
Combustion
Слайд 17
Phytoremediation vs.
Mechanical/chemical treatment
Cheaper
Advantages of phytoremediation
~10 -
100x
Excavation & reburial: up to $1 million/acre
Revegetation: ~$20,000/acre
Слайд 18
Phytoremediation vs.
Mechanical/chemical treatment
Advantages of phytoremediation (cont.)
Less
intrusive
Can be more permanent solution
Better public acceptance
Слайд 19
Limitations of phytoremediation
Phytoremediation vs.
Mechanical/chemical treatment (cont.)
Can
be slower
Limited by rate of biological processes
-
Metabolic breakdown (organics): fairly fast- Filter action by plants: fast (days)
Accumulation in plant tissue: slow
e.g. metals: average 15 yrs to clean up site
(< 1yr)
Слайд 20
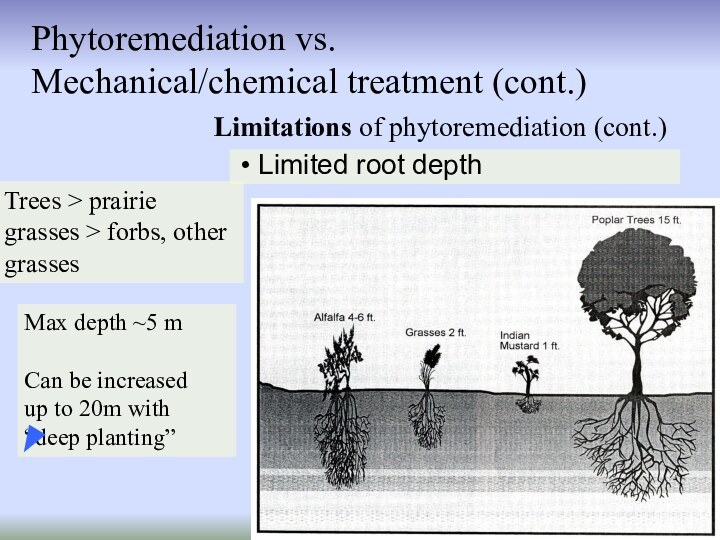
Limitations of phytoremediation (cont.)
Phytoremediation vs.
Mechanical/chemical treatment (cont.)
Limited root depth
Trees > prairie grasses > forbs, other
grasses
Слайд 21
Limitations of phytoremediation (cont.)
Phytoremediation vs.
Mechanical/chemical treatment (cont.)
Plant tolerance to pollutant/conditions
Bioavailability of contaminant
- Bigger problem
with metals than organics- Can be alleviated using amendments, or treating hot spots by other method
- Bioavailability can be enhanced by amendments
Слайд 22
So, when choose phytoremediation?
Sufficient time available
Pollution shallow enough
Pollutant concentrations not phytotoxic
For very large
quantities of mildly contaminated substrate:
phytoremediation only cost-effective option
Note: Phyto may be used in conjunction with
other remediation methods
$$ limited
Слайд 23
Techniques/strategies of phytoremediation
phytoextraction (or phytoaccumulation),
phytostabilization,
Phytostimulation,
phytofiltration,
phytovolatilization,
and phytodegradation
Слайд 24
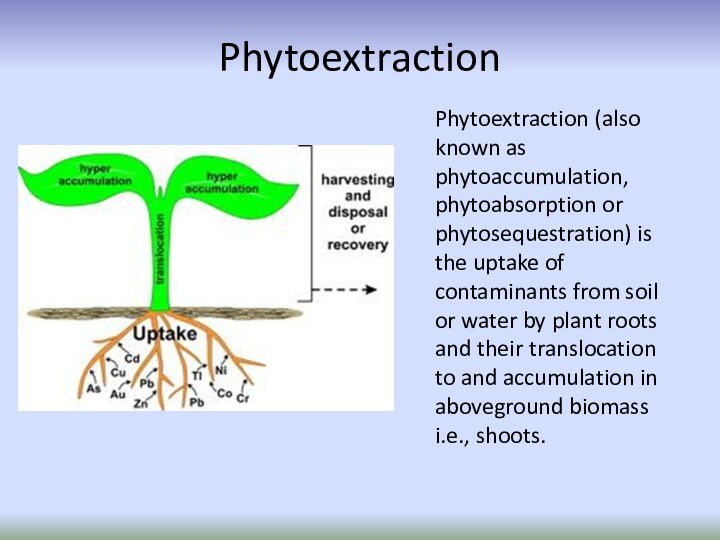
Phytoextraction
Phytoextraction (also known as phytoaccumulation, phytoabsorption or phytosequestration)
is the uptake of contaminants from soil or water
by plant roots and their translocation to and accumulation in aboveground biomass i.e., shoots.
Слайд 27
Phytostabilization
Phytostabilization or phytoimmobilization is the use of
certain plants for stabilization of contaminants in contaminated soils
is
used to reduce the mobility and bioavailability of pollutants in the environment, thus preventing their migration to groundwater or their entry into the food chain.Plants can immobilize heavy metals in soils through:
sorption by roots,
precipitation,
complexation or metal valence reduction in rhizosphere etc.
Слайд 33
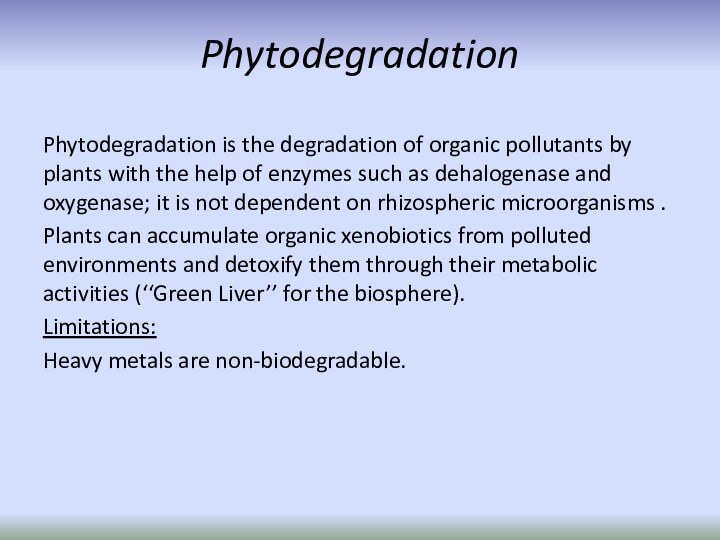
Phytodegradation
Phytodegradation is the degradation of organic pollutants
by plants with the help of enzymes such as
dehalogenase and oxygenase; it is not dependent on rhizospheric microorganisms .Plants can accumulate organic xenobiotics from polluted environments and detoxify them through their metabolic activities (‘‘Green Liver’’ for the biosphere).
Limitations:
Heavy metals are non-biodegradable.
Слайд 35
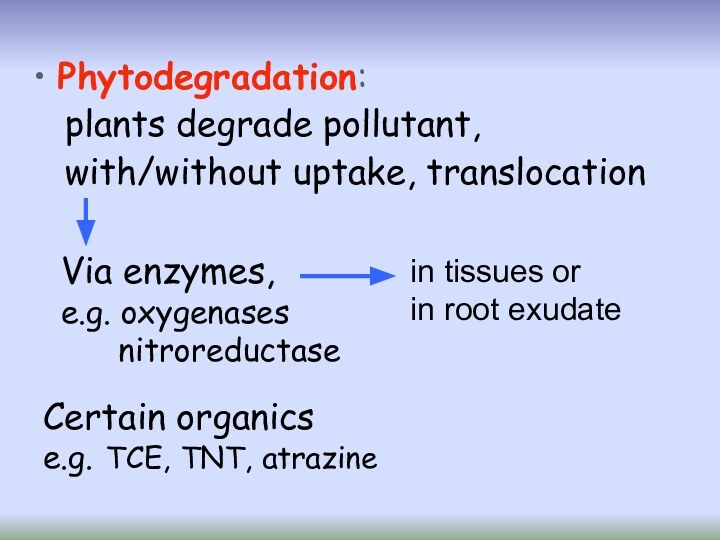
Phytodegradation:
plants degrade pollutant,
with/without uptake, translocation
Certain
organics
e.g. TCE, TNT, atrazine
Слайд 36
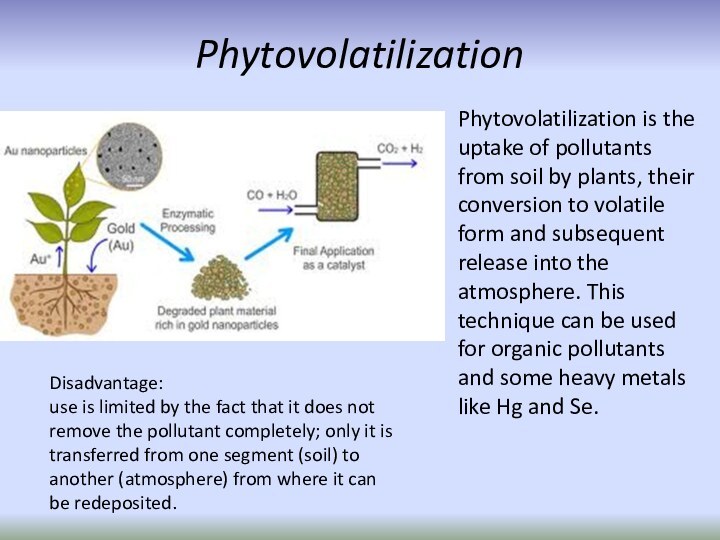
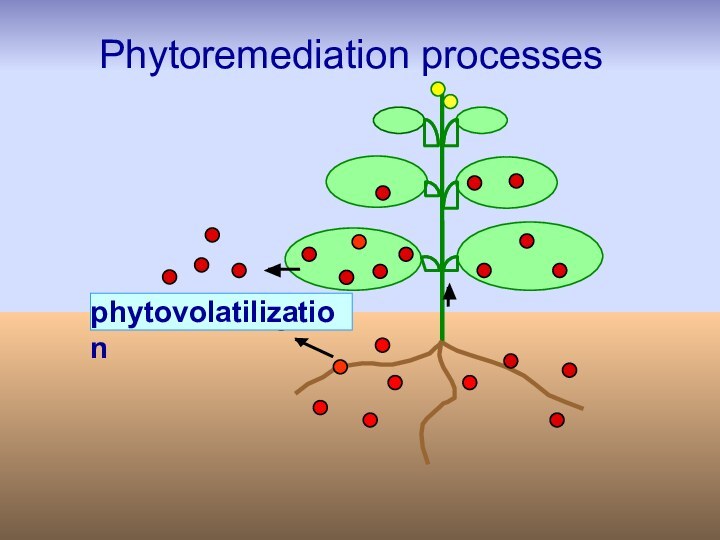
Phytovolatilization
Phytovolatilization is the uptake of pollutants from
soil by plants, their conversion to volatile form and
subsequent release into the atmosphere. This technique can be used for organic pollutants and some heavy metals like Hg and Se.Disadvantage:
use is limited by the fact that it does not remove the pollutant completely; only it is transferred from one segment (soil) to another (atmosphere) from where it can be redeposited.
Слайд 39
Rhizodegradation
Rhizodegradation refers to the breakdown of organic pollutants
in the soil by microorganisms in the rhizosphere. Rhizosphere
extends about 1 mm around the root and is under the influence of the plant.Plants can stimulate microbial activity about 10–100 times higher in the rhizosphere by the secretion of exudates containing carbohydrates, amino acids, flavonoids.
The release of nutrients-containing exudates by plant roots provides carbon and nitrogen sources to the soil microbes and creates a nutrient-rich environment in which microbial activity is stimulated.
Слайд 41
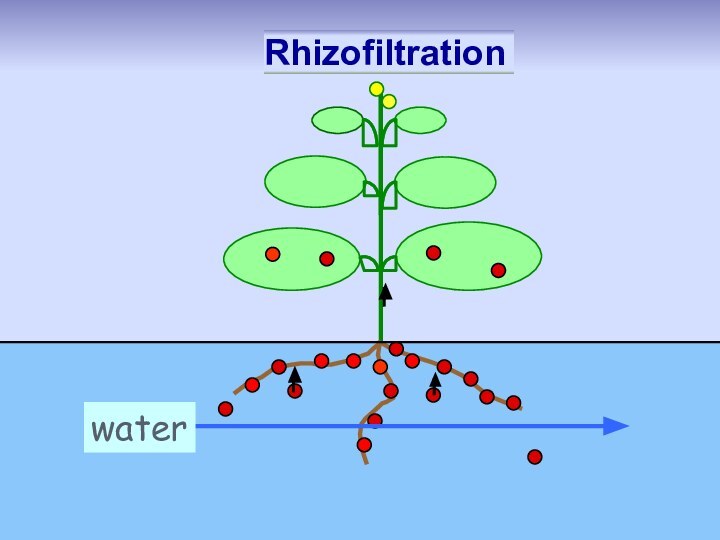
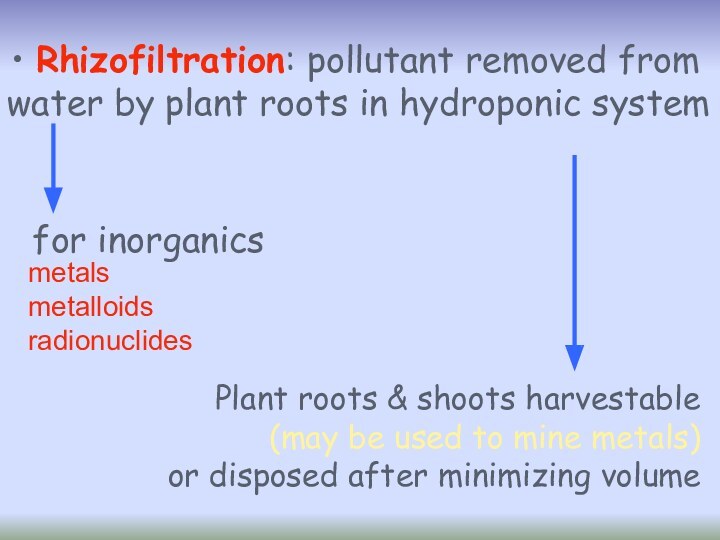
Rhizofiltration: pollutant removed from
water by plant
roots in hydroponic system
metals
metalloids
radionuclides
Слайд 42
Phytofiltration
Phytofiltration is the removal of pollutants from
contaminated surface waters or waste waters by plants.
Phytofiltration may
be:rhizofiltration (use of plant roots);
blastofiltration (use of seedlings) or caulofiltration (use of excised plant shoots; Latin caulis = shoot)
Слайд 43
Rhizofiltration
Hydroponics for metal remediation:
75% of metals removed
from mine drainage
Involves:
phytoextraction
phytostabilization
Слайд 44
Constructed wetland for Se remediation:
Involves:
phytoextraction
phytovolatilization
phytostabilization
(rhizofiltration)
(phytostimulation)
75% of Se removed from ag
drainage water
Слайд 45
Phytodesalination
Phytodesalination refers to the use of halophytic
plants for removal of salts from salt-affected soils in
order to enable them for supporting normal plant growth.
Слайд 46
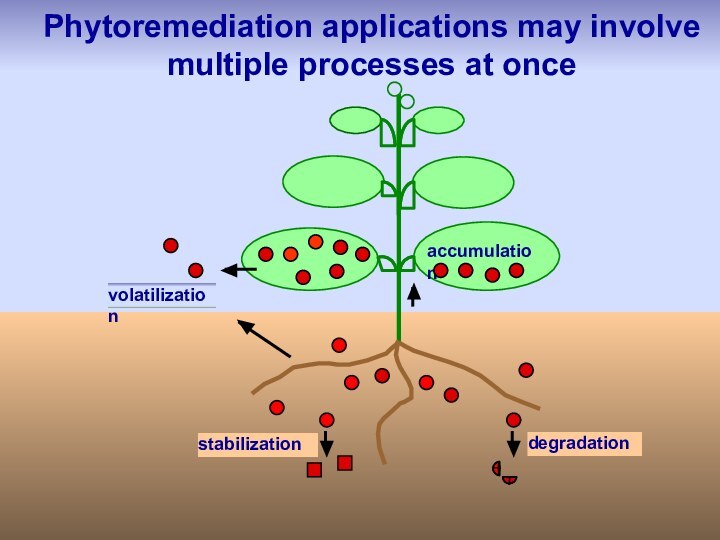
stabilization
degradation
volatilization
accumulation
Phytoremediation applications may involve
multiple processes at once
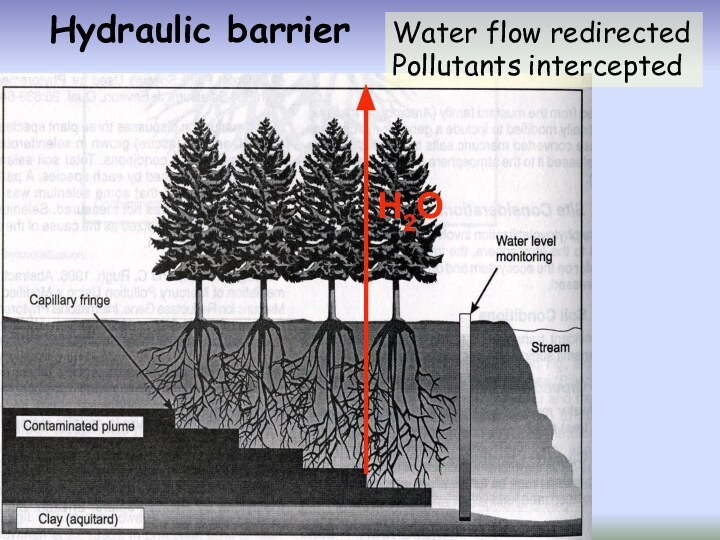
Слайд 48
Natural attenuation: polluted site left alone
but
monitored
Vegetative cap: polluted site revegetated,
then left alone, monitoredСлайд 51 heavy metals originate from extraction of ores and
processing
heavy metals are non-biodegradable,
they accumulate in the
environment subsequently contaminate the food chain.
heavy metals cause toxicological effects on soil microbes, which may lead to a decrease in their numbers and activities
This contamination poses a risk to environmental and human health.
Essential HM: Fe, Mn, Cu, Zn, and Ni
Non-essential HM: Cd, Pb, As, Hg, and Cr.
Heavy metals & organic compounds
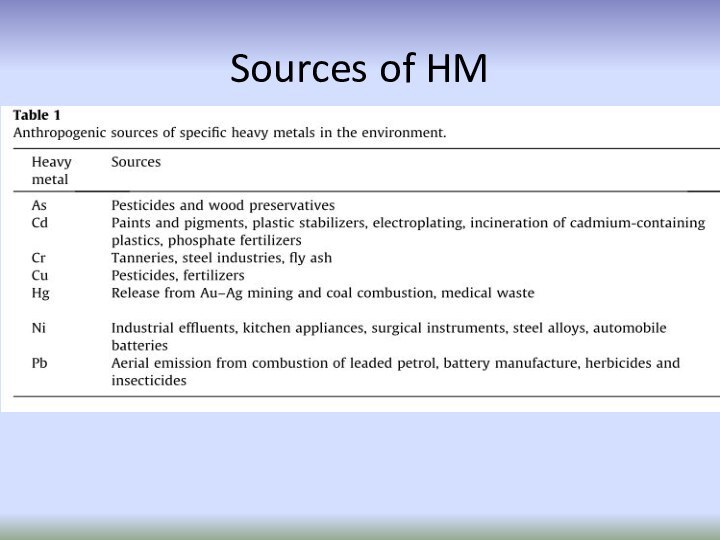
Слайд 52
Anthropogenic sources
Sources of heavy metals in the environment
Natural sources
weathering of minerals,
erosion and volcanic activity
mining,
smelting,
electroplating,
use of pesticides and (phosphate)
fertilizers as well as biosolids in agriculture,
sludge dumping,
industrial discharge,
atmospheric deposition, etc.
Слайд 54 Harmful effects of heavy metals on human health
are toxic and can cause undesirable effects and severe
problems even at very low concentrationscause oxidative stress
can replace essential metals in pigments or enzymes disrupting their function
the most problematic heavy metals are Hg, Cd, Pb, As, Cu, Zn, Sn, and Cr
Слайд 56
Cleanup of heavy metal-contaminated soils
Cleanup of heavy metal-contaminated
soils is utmost necessary in order to minimize their
impact on the ecosystems.The conventional remediation methods include in situ vitrification, soil incineration, excavation and landfill, soil washing, soil flushing, solidification, and stabilization of electro-kinetic systems
Disadvantages: high costs, intensive labor, irreversible changes in soil properties and disturbance of native soil microflora, secondary pollution etc.
Слайд 57 Phytoremediation – a green solution to the HM
problem
‘‘Phytoremediation basically refers to the use of plants
and associated soil microbes to reduce the concentrations or toxic effects of contaminants in the environments’’ (Greipsson, 2011).It can be used for removal of heavy metals and radionuclides as well as for organic pollutants (such as, polynuclear aromatic hydrocarbons, polychlorinated biphenyls, and pesticides).
It is a novel, cost-effective, efficient, environment- and eco-friendly, in situ applicable, and solar-driven remediation strategy.
Plants generally handle the contaminants without affecting topsoil, uptake pollutants from the environment .
low installation and maintenance costs.
The establishment of vegetation on polluted soils also helps prevent erosion and metal leaching
Слайд 58
Purpose of phytoremediation
risk containment (phytostabilization);
phytoextraction of metals
with market value such as Ni, Tl and Au;
durable land management where phytoextraction gradually improves soil quality for subsequent cultivation of crops with higher market value.
Furthermore, fast-growing and high-biomass producing plants such as willow, poplar and Jatropha could be used for both phytoremediation and energy production.
Слайд 59
Phytoextraction of heavy metals
The main and most
useful phytoremediation technique for removal of HM and metalloids
from polluted soils, sediments or water. The efficiency depends on many factors like bioavailability of the heavy metals in soil, soil properties, speciation of the heavy metals and plant species concerned. Plants suitable for phytoextraction should ideally have the following characteristics:High growth rate.
Production of more above-ground biomass.
Widely distributed and highly branched root system.
More accumulation of the target heavy metals from soil.
Translocation of the accumulated heavy metals from roots to shoots.
Tolerance to the toxic effects of the target heavy metals.
Good adaptation to prevailing environmental and climatic conditions.
Resistance to pathogens and pests.
Easy cultivation and harvest.
Repulsion to herbivores to avoid food chain contamination.
Слайд 60
Phytoextraction: two key factors
The phytoextraction potential of
a plant species is mainly determined by two key
factors i.e., shoot metal concentration and shoot biomass. Two different approaches have been tested for phytoextraction of heavy metals:(1) The use of hyperaccumulators, which produce comparatively less aboveground biomass but accumulate target heavy metals to a greater extent;
(2) The application of other plants, such as Brassica juncea (Indian mustard), which accumulate target heavy metals to a lesser extent but produce more aboveground biomass so that overall accumulation is comparable to that of hyperaccumulators due to production of more biomass.
Слайд 61
Bioavailability of HM in soils
Chemical composition and sorption
properties of soil influence the mobility and bioavailability of
metals. Low bioavailability is a major limiting factor for phytoextraction of contaminants. Strong binding of heavy metals to soil particles or precipitation causes a significant fraction of soil heavy metals insoluble and therefore mainly unavailable for uptake by plants.Bioavailability of heavy metals/metalloids in soil:
readily bioavailable (Cd, Ni, Zn, As, Se, Cu);
moderately bioavailable (Co, Mn, Fe)
and least bioavailable (Pb, Cr, U)
However, plants have developed certain mechanisms for solubilizing heavy metals in soil. Plant roots secrete metal-mobilizing substances in the rhizosphere called phytosiderophores . Secretion of H+ ions by roots can acidify the rhizosphere and increase metal dissolution. H+ ions can displace heavy metal cations adsorbed to soil particles
Слайд 62
Phytoextraction: two modes
Natural conditions: no soil amendm.
Induced or
chelate assisted phytoextraction: different chelating agents such as EDTA
(etylendiamintetraacetic acid), citric acid, elemental sulfur, and (NH4)2SO4 are added to soil to increase the bioavailability of heavy metals in soil for uptake by plants.Bioavailability of the heavy metals can also be increased by lowering soil pH since metal salts are soluble in acidic media rather than in basic media. However, these chemical treatments can cause secondary pollution problems.
Use of citric acid as a chelating agent could be promising because it has a natural origin and is easily biodegraded in soil.
Слайд 63
Metallophytes
Metallophytes are plants that are specifically adapted to
and thrive in heavy metal-rich soils.
Metallophytes are divided into
three categories: 1. Metal excluders accumulate heavy metals from substrate into their roots but restrict their transport and entry into their aerial parts. Such plants have a low potential for metal extraction but may be efficient for phytostabilization purposes.,
2. Metal indicators accumulate heavy metals in their aerial parts and reflect heavy metal concentrations in the substrate
3. Metal hyperaccumulators are plants, which can concentrate heavy metals in their aboveground tissues to levels far exceeding those present in the soils or non-accumulating plants. These plants are concentrated in the plant family Brassicaceae. Their use especially in mining regions, either alone or in combination with microorganisms, for phytoremediation of heavy metal-contaminated soils is an attractive idea.
Слайд 64
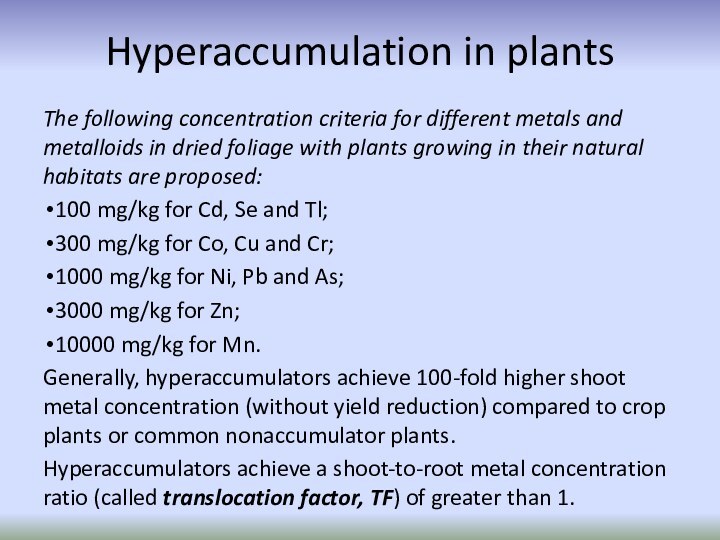
Hyperaccumulation in plants
The following concentration criteria for different
metals and metalloids in dried foliage with plants growing
in their natural habitats are proposed:100 mg/kg for Cd, Se and Tl;
300 mg/kg for Co, Cu and Cr;
1000 mg/kg for Ni, Pb and As;
3000 mg/kg for Zn;
10000 mg/kg for Mn.
Generally, hyperaccumulators achieve 100-fold higher shoot metal concentration (without yield reduction) compared to crop plants or common nonaccumulator plants.
Hyperaccumulators achieve a shoot-to-root metal concentration ratio (called translocation factor, TF) of greater than 1.
Слайд 65
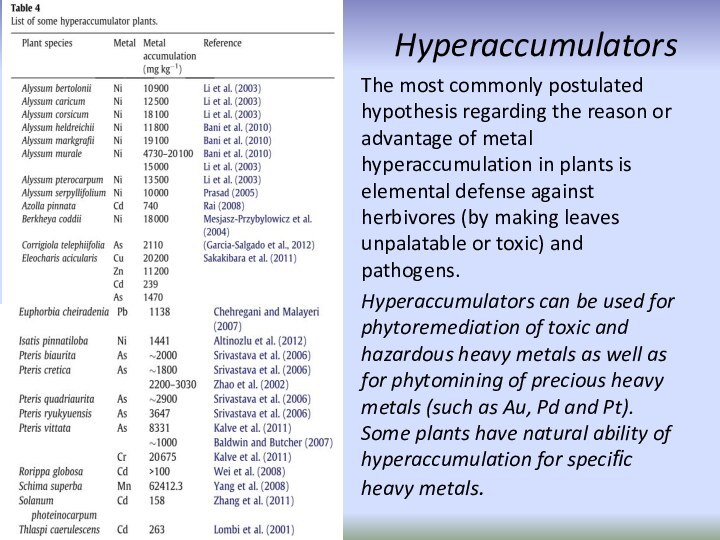
Hyperaccumulators
The most commonly postulated hypothesis regarding the reason
or advantage of metal hyperaccumulation in plants is elemental
defense against herbivores (by making leaves unpalatable or toxic) and pathogens.Hyperaccumulators can be used for phytoremediation of toxic and hazardous heavy metals as well as for phytomining of precious heavy metals (such as Au, Pd and Pt). Some plants have natural ability of hyperaccumulation for specific heavy metals.
Слайд 66
Quantification of phytoextraction efficiency
Bioconcentration factor indicates the efficiency
of a plant species in accumulating a metal into
its tissues from the surrounding environment. It is calculated as followswhere Charvested tissue is the concentration of the target metal in the plant harvested tissue and Csoil is the concentration of the same metal in the soil (substrate).
Translocation factor indicates the efficiency of the plant in translocating the accumulated metal from its roots to shoots. It is calculated as follows
where Cshoot is concentration of the metal in plant shoots and Croot is concentration of the metal in plant roots.
Слайд 67
Quantification of phytoextraction efficiency
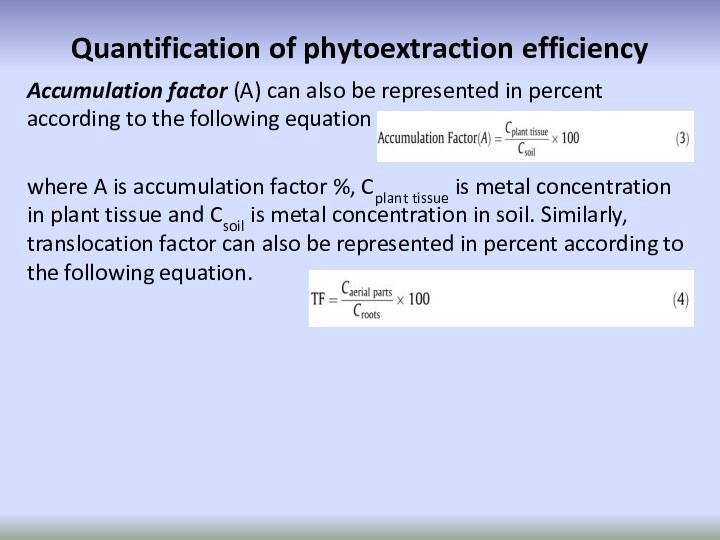
Accumulation factor (A) can also
be represented in percent according to the following equation
where
A is accumulation factor %, Cplant tissue is metal concentration in plant tissue and Csoil is metal concentration in soil. Similarly, translocation factor can also be represented in percent according to the following equation.
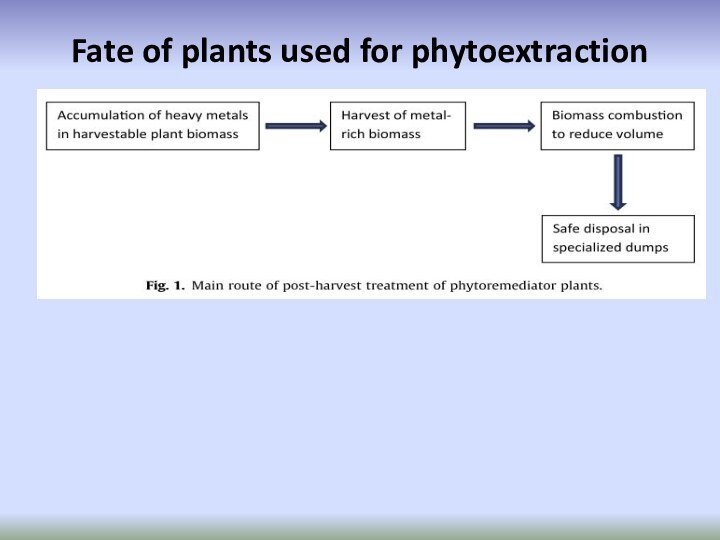
Слайд 69
Phytomining
Advantages:
- can be combusted to get energy and
the remaining ash is considered as ‘‘bio-ore’’;
phytomining is the
sale of energy from combustion of the biomass;bio-ore can be processed for the recovery or extraction of the heavy metals;
Processing bio-ores contributes less SOx emissions to the atmosphere;
Phytomining has been commercially used for Ni and it is believed that it is less expensive than the conventional extraction methods.
Слайд 70
Use of constructed wetlands for phytoremediation
Constructed wetlands are
used for clean-up of effluents and drainage waters. Aquatic
macrophytes are more suitable for wastewater treatment than terrestrial plants due to their faster growth, production of more biomass and relative higher ability of pollutant uptake.Poplar (Populus spp.) and willow (Salix spp.) can be used on the edge. Water hyacinth (Eichhornia crassipes) has been used for phytoremediation of heavy metals at constructed wetlands. Water lettuce (Pistia stratiotes) has been pointed out as a potential phytoremediator plant for Mn contaminated waters. Azolla (short doubling time 2–3 d) has nitrogen fixation ability and tolerance to and accumulation of a wide range of heavy metals.
Слайд 71
Mechanism of heavy metals’ uptake, translocation, and tolerance
Plants
take heavy metals from soil solution into their roots.
After entry into roots, heavy metal ions can either be stored in the roots or translocated to the shoots primarily through xylem vessels where they are mostly deposited in vacuoles.The mechanism of phytoextraction of heavy metals has five basic aspects:
mobilization of the heavy metals in soil,
uptake of the metal ions by plant roots,
translocation of the accumulated metals from roots to aerial tissues,
sequestration of the metal ions in plant tissues
and metal tolerance.
Mechanisms governing heavy metal tolerance in plant cells are cell wall binding, active transport of ions into the vacuole and chelation through the induction of metal-binding peptides and the formation of metal complexes. Organic acids and amino acids are suggested as ligands for chelation of heavy metal ions because of the presence of donor atoms (S, N, and O) in their molecules.
Слайд 72
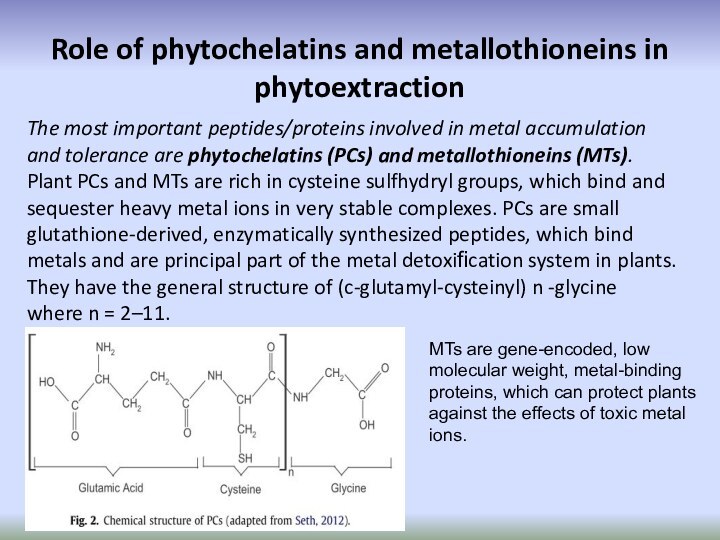
Role of phytochelatins and metallothioneins in phytoextraction
The
most important peptides/proteins involved in metal accumulation and tolerance
are phytochelatins (PCs) and metallothioneins (MTs). Plant PCs and MTs are rich in cysteine sulfhydryl groups, which bind and sequester heavy metal ions in very stable complexes. PCs are small glutathione-derived, enzymatically synthesized peptides, which bind metals and are principal part of the metal detoxification system in plants. They have the general structure of (c-glutamyl-cysteinyl) n -glycine where n = 2–11.MTs are gene-encoded, low molecular weight, metal-binding proteins, which can protect plants against the effects of toxic metal ions.
Слайд 73
Limitations of phytoremediation
Long time required
Hyperaccumulators are usually
limited by their slow growth rate and low biomass
limited
bioavailability of tightly bound fraction of metal ions from soil It is applicable to sites with low to moderate levels of metal contamination
Risk of food chain contamination
Слайд 74
Future trends in phytoremediation
Phytoremediation is a relatively recent
field of research. Results in actual field can be
different from those at laboratory or greenhouse conditions (different factors simultaneously play their role).Factors that may affect phytoremediation in the field include:
variations in temperature,
nutrients,
precipitation and moisture,
plant pathogens and herbivory,
uneven distribution of contaminants,
soil type,
soil pH,
soil structure etc.
Слайд 75
Future challenges in phytoremediation
Phytoremediation efficiency of different plants
for specific target heavy metals has to be tested
in field conditions in order to realize the feasibility of this technology for commercialization.Identification of desirable traits in natural hyperaccumulators --- selection and breeding techniques. Thus different desirable traits can be combined into a single plant species.
In spite of the many challenges, phytoremediation is perceived as a green remediation technology with an expected great potential.
Слайд 77
Conclusions
Physical and chemical methods for clean-up and restoration
of heavy metal-contaminated soils have serious limitations like high
cost, irreversible changes in soil properties, destruction of native soil microflora and creation of secondary pollution problems.In contrast, phytoremediation is environment-friendly and ecologically responsible solar-driven technology with good public acceptance.
phytomining – a plant-based eco-friendly mining of metals, which can be used for extraction of metals even from low-grade ores.
Phytoextraction of heavy metals is expected to be a commercially viable technology for phytoremediation and phytomining of heavy metals in future.