- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Hydrophilization and hydrophobization of the surface of solids with the help of SAA
Содержание
- 2. What is hydrophobization of surfaces?Hydrophobization of surfaces
- 3. What is hydrophilization of surfaces?Hydrophilization of the
- 4. Hydrophilization of surfaces by surfactantsWhen the surface
- 5. Hydrophobization of surface by surfactant The effect
- 6. Adsorption of SAA on solid surfaceSAA- surface
- 7. Adsorption phenomena in the case of solids
- 8. In accordance with the rule of the
- 9. Adsorption of surfactants on solid particles from
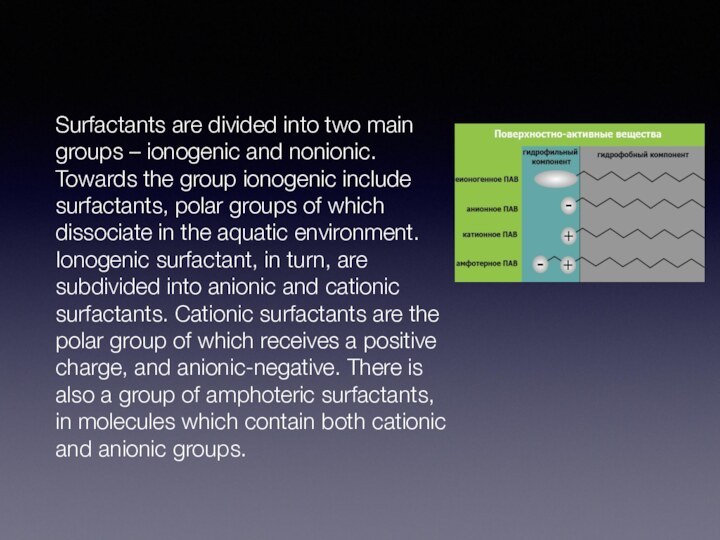
- 10. Surfactants are divided into two main groups
- 11. Скачать презентацию
- 12. Похожие презентации
What is hydrophobization of surfaces?Hydrophobization of surfaces By hydrophobization it is customary to call a process in which the ability of materials to be wetted by water (aqueous solutions) is sharply reduced, and the vapor and










Слайд 3
What is hydrophilization of surfaces?
Hydrophilization of the surface
means a stable significant increase in polarity, which persists
even after the evaporation of water. Such a change in polarity is achieved by a more radical interference in the nature of the surface layer when the chemical composition of the surface macromolecules changes.The surface hydrophilization also reduces the average adhesion force, but to a lesser extent than hydrophobization. A similar decrease in the average adhesion strength during hydrophilization is observed in the air.
Слайд 4
Hydrophilization of surfaces by surfactants
When the surface is
hydrophilized under the influence of surfactant, the wetting work
increases with increasing surfactant concentration in the solution. This indicates a decrease in the interfacial tension at the T-G interface due to adsorption and an increase in the affinity of the liquid to a given surface.Surface-active substances are chemical compounds, which, concentrating on the interface of thermodynamic phases, cause a decrease in surface tension
Слайд 5
Hydrophobization of surface by surfactant
The effect of
hydrophobization is based on the adsorption of surfactants on
the surface of the rock, improving the wettability of its oil and, consequently, increasing the phase permeability for oil. This contributes to an increase in the flow rate of oil and a reduction in the water cut of the extracted products.
Слайд 6
Adsorption of SAA on solid surface
SAA- surface active
agents. e adsorption of these molecules at the solid-liquid
interface depends on several factors such as the nature of the substrate, solvent, adsorbate species, the presence of secondary competing-cooperative species, temperature and even mode of mixing.
Слайд 7
Adsorption phenomena in the case of solids are
usually studied on powders, S specific surface area which
is determined independently (usually by adsorptiongases'.) In this case, as a rule, determine the number of substances Г* absorbed by the unit of mass of powder, Г*= ∆сV/M1where ∆с –the change of concentration in volume of solution V, M1- mass of powder. Here value of absorption equals to
Г= Г*/S
Слайд 8
In accordance with the rule of the greatest
adsorption polarity equalizationability to have a surfactant with an
intermediate (between solid and liquid)polarity. An essential feature of adsorption on solids is the possibility ofthe formation of chemical bonds between surfactant molecules and a solid.We use SAA for:
for hydrophilization and hydrophobication solid surfaces.
process control wetting and selective wettings.