Слайд 2
Gene Expression Systems in Prokaryotes and Eukaryotes
Expression studies:
1. Analyzing Transcription
- Northern
blot
- Micro array
- real-time PCR
- Primer extension
2. In vivo Expresion studies
Use of report genes to study regulatory elements
3. Analyzing Translation
- Western blot - immuno assays
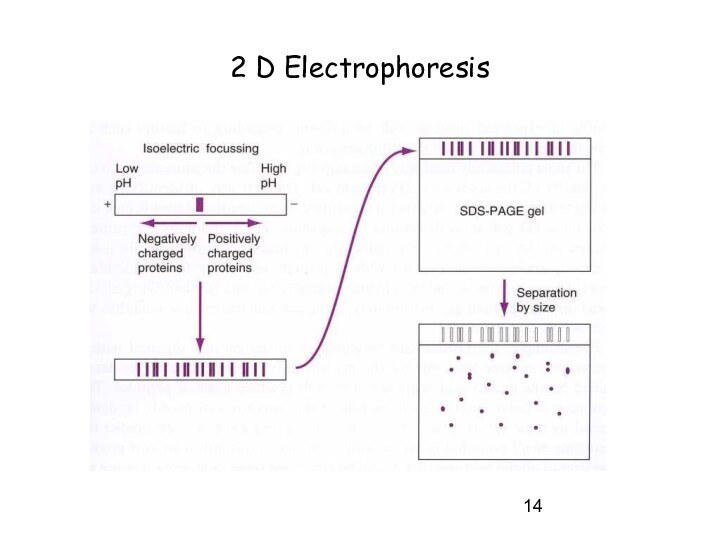
- 2D electrophoresis
- proteomics
Слайд 3
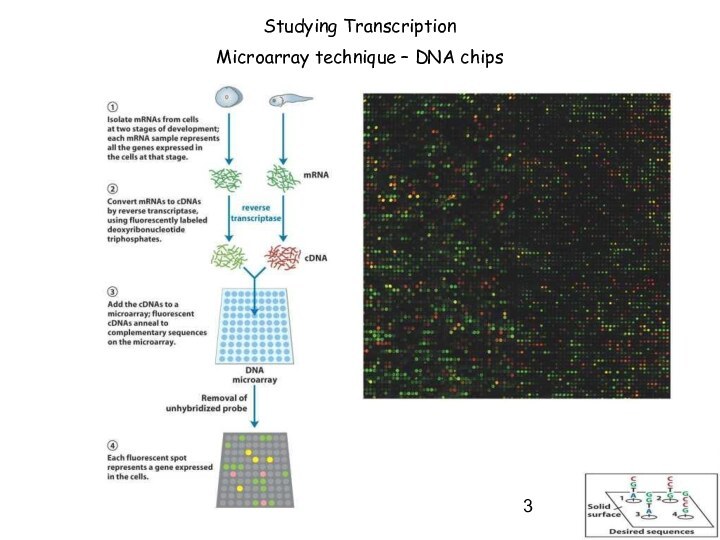
Studying Transcription
Microarray technique – DNA chips
Слайд 5
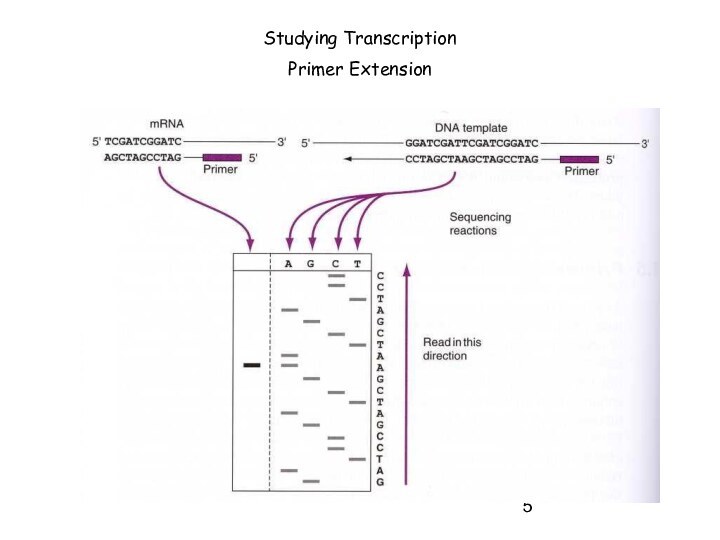
Studying Transcription
Primer Extension
Слайд 6
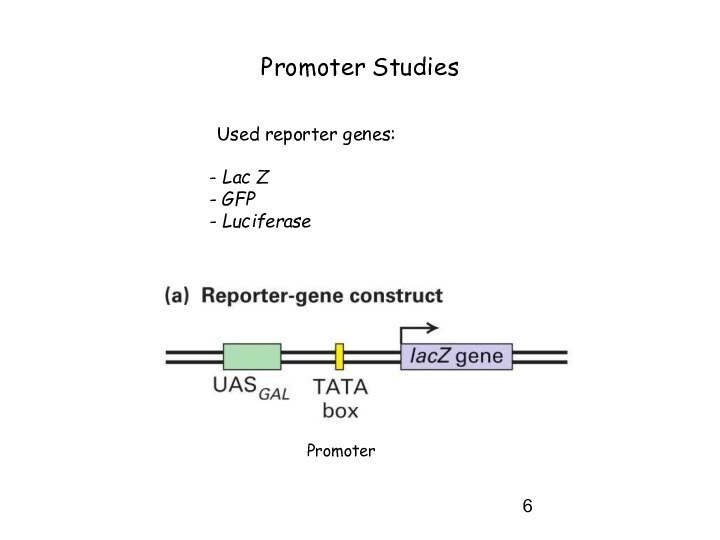
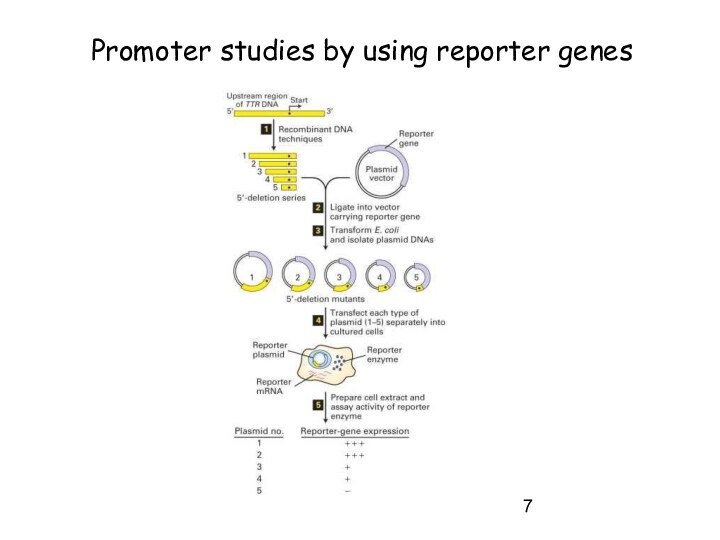
Promoter Studies
Used reporter genes:
Lac Z
GFP
Luciferase
Promoter
Слайд 7
Promoter studies by using reporter genes
Слайд 8
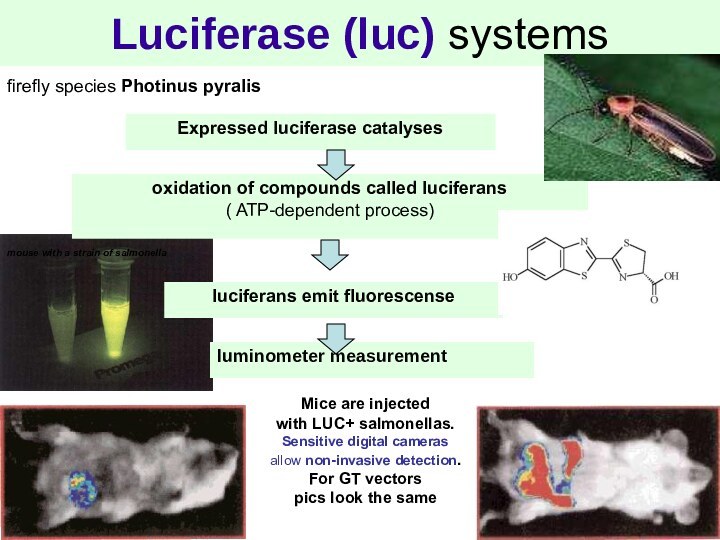
Luciferase (luc) systems
firefly species Photinus pyralis
oxidation of
compounds called luciferans
( ATP-dependent process)
luciferans emit fluorescense
Expressed luciferase catalyses
mouse
with a strain of salmonella
Mice are injected
with LUC+ salmonellas.
Sensitive digital cameras
allow non-invasive detection.
For GT vectors
pics look the same
luminometer measurement
Слайд 9
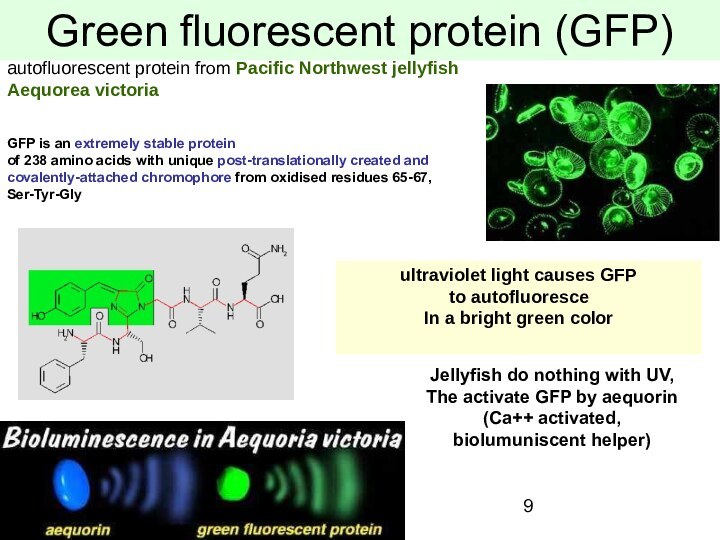
Green fluorescent protein (GFP)
autofluorescent protein from Pacific Northwest
jellyfish
Aequorea victoria
ultraviolet light causes GFP
to autofluoresce
In
a bright green color
Jellyfish do nothing with UV,
The activate GFP by aequorin
(Ca++ activated,
biolumuniscent helper)
Слайд 10
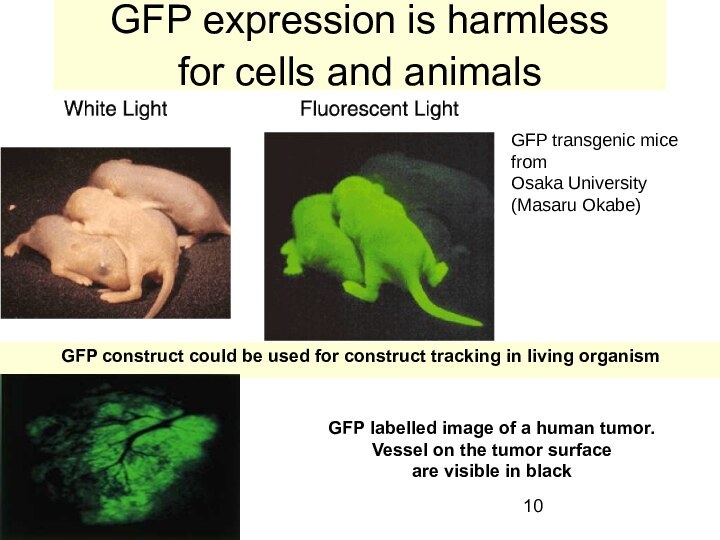
GFP expression is harmless
for cells and animals
GFP
transgenic mice from
Osaka University
(Masaru Okabe)
GFP construct could
be used for construct tracking in living organism
GFP labelled image of a human tumor.
Vessel on the tumor surface
are visible in black
Слайд 11
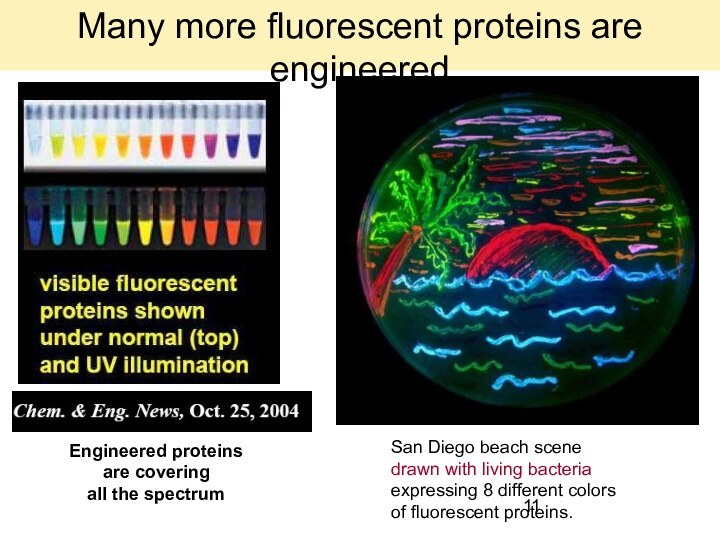
Engineered proteins
are covering
all the spectrum
San Diego
beach scene
drawn with living bacteria
expressing 8 different
colors
of fluorescent proteins.
Many more fluorescent proteins are engineered
Слайд 12
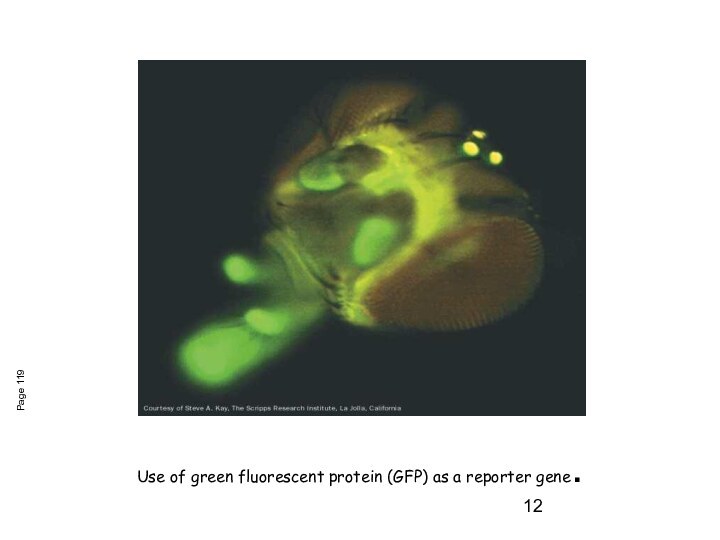
Use of green fluorescent protein (GFP) as a
reporter gene.
Page 119
Слайд 13
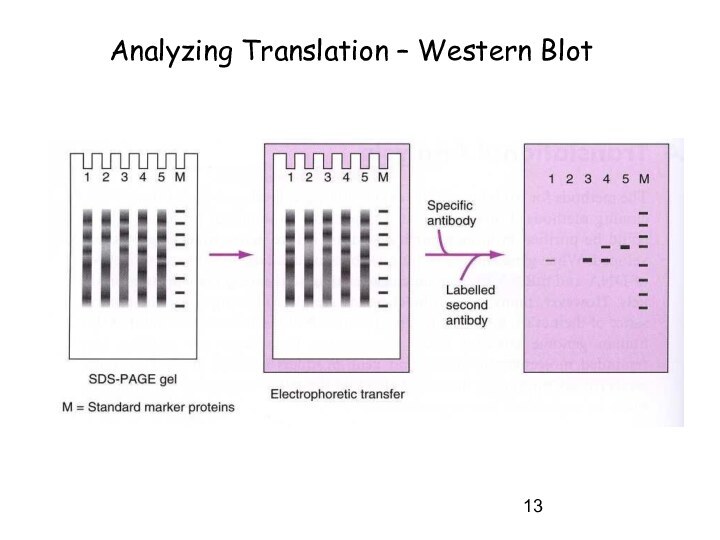
Analyzing Translation – Western Blot
Слайд 15
Gene Expression
Transcriptional start
Translational start
Слайд 16
Gene Expression
Gene copy number:
1. Plasmid copy
number:
The copy-number of a plasmid in
the cell is determined by regulating the initiation of plasmid replication.
The initiation of plasmid replication may be controlled by:
the amount of available primer (RNA)
the amount of essential replication proteins
the function of essential replication proteins.
2. Gene dosage -> number of genes integrated into chromosome
- prokaryotic systems -> i.e. Transposons, phages, recombinantion
- mainly eukaryotic systems
Слайд 17
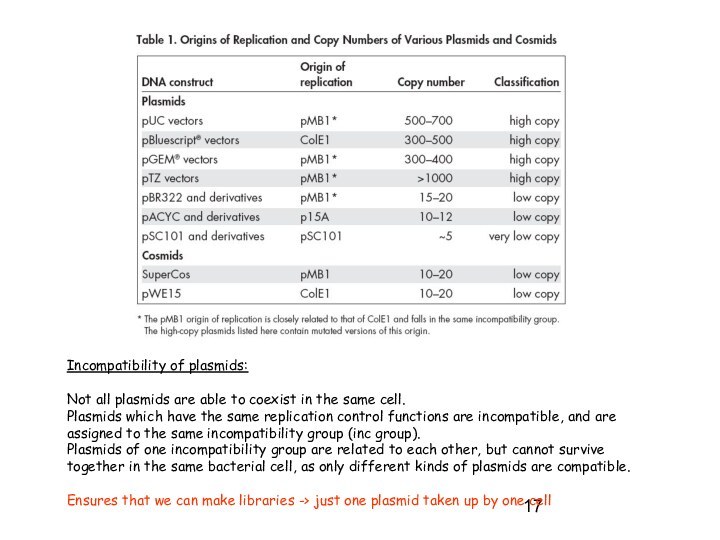
Incompatibility of plasmids:
Not all plasmids are able to
coexist in the same cell.
Plasmids which have the
same replication control functions are incompatible, and are assigned to the same incompatibility group (inc group).
Plasmids of one incompatibility group are related to each other, but cannot survive together in the same bacterial cell, as only different kinds of plasmids are compatible.
Ensures that we can make libraries -> just one plasmid taken up by one cell
Слайд 18
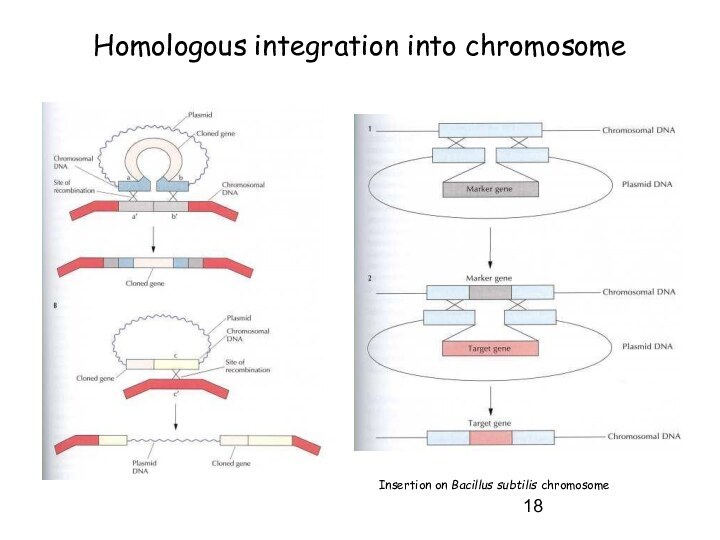
Homologous integration into chromosome
Insertion on Bacillus subtilis chromosome
Слайд 19
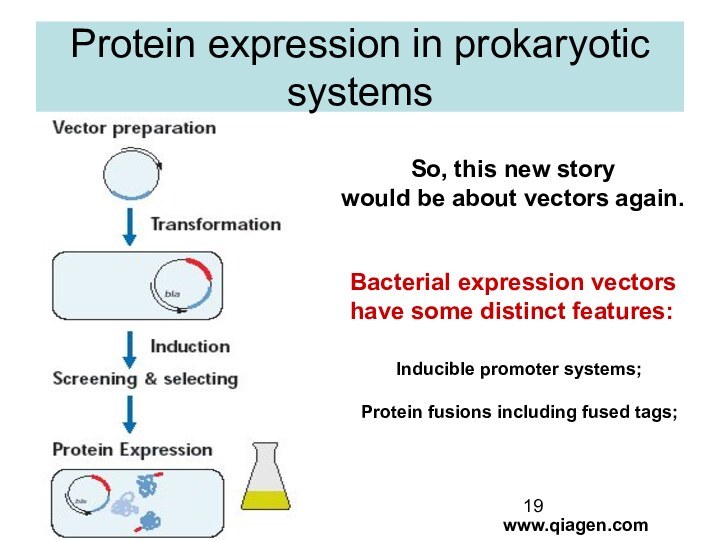
Protein expression in prokaryotic systems
www.qiagen.com
So, this new story
would be about vectors again.
Bacterial expression vectors
have
some distinct features:
Inducible promoter systems;
Protein fusions including fused tags;
Слайд 20
General advices for one who wants
to produce
gene expression
in prokaryotes
1. Do not forget to cut
out the intron
2. Check orientation of insert
3. Do fusions with something In-frame
Most obvious and common mistakes:
4. No Post-translation modification
= no product activity
Слайд 21
www.wzw.tum.dewww.wzw.tum.de/gene-quantification/ www.wzw.tum.de/gene-quantification/ mrna.html
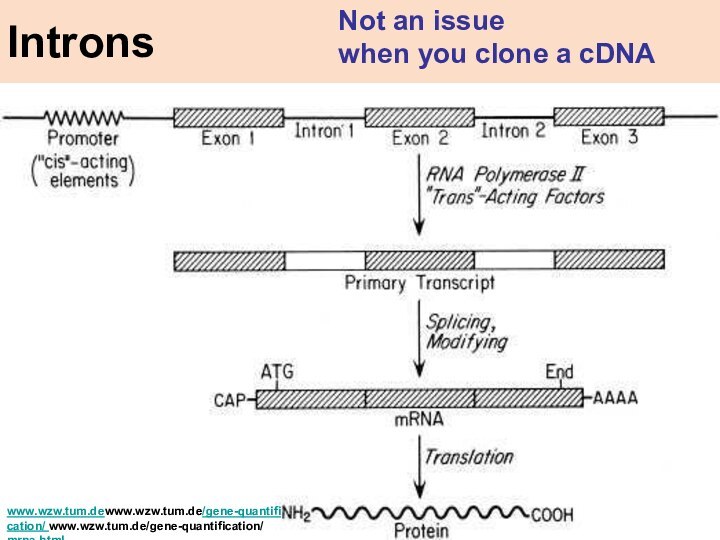
Introns
Not an issue
when you clone
a cDNA
Слайд 22
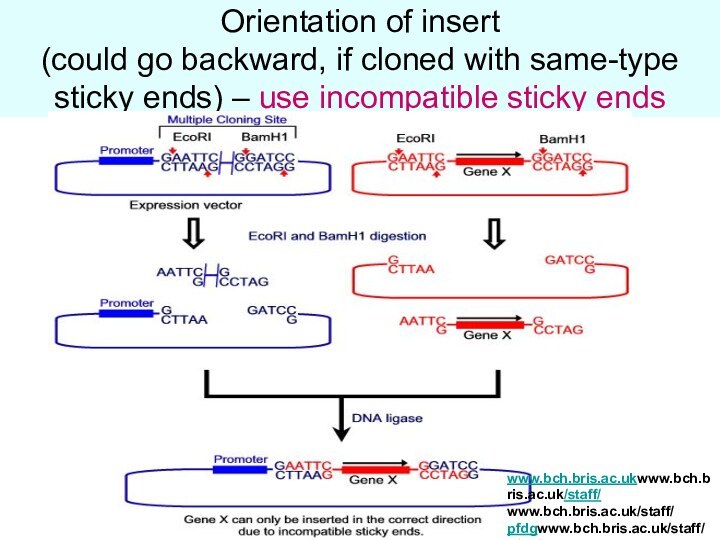
Orientation of insert
(could go backward, if cloned
with same-type sticky ends) – use incompatible sticky ends
www.bch.bris.ac.ukwww.bch.bris.ac.uk/staff/
www.bch.bris.ac.uk/staff/ pfdgwww.bch.bris.ac.uk/staff/ pfdg/
teaching/teaching/genes.htm
Слайд 23
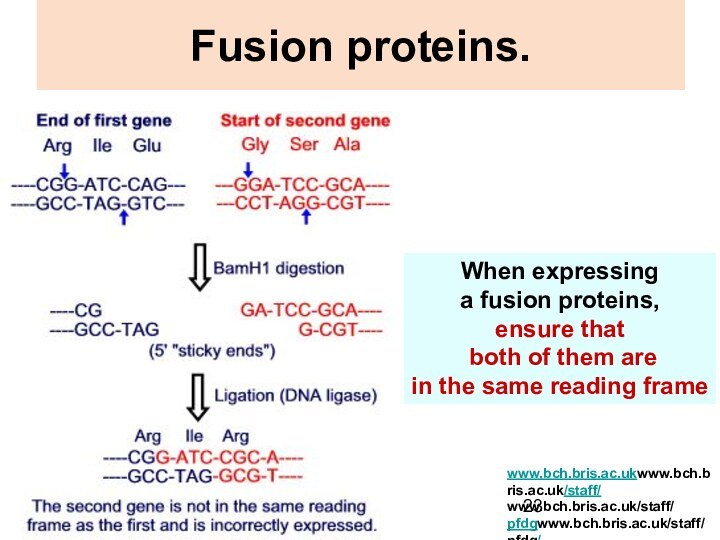
Fusion proteins.
When expressing
a fusion proteins,
ensure that
both of them are
in the same reading frame
www.bch.bris.ac.ukwww.bch.bris.ac.uk/staff/ www.bch.bris.ac.uk/staff/
pfdgwww.bch.bris.ac.uk/staff/ pfdg/
teaching/teaching/genes.htm
Слайд 24
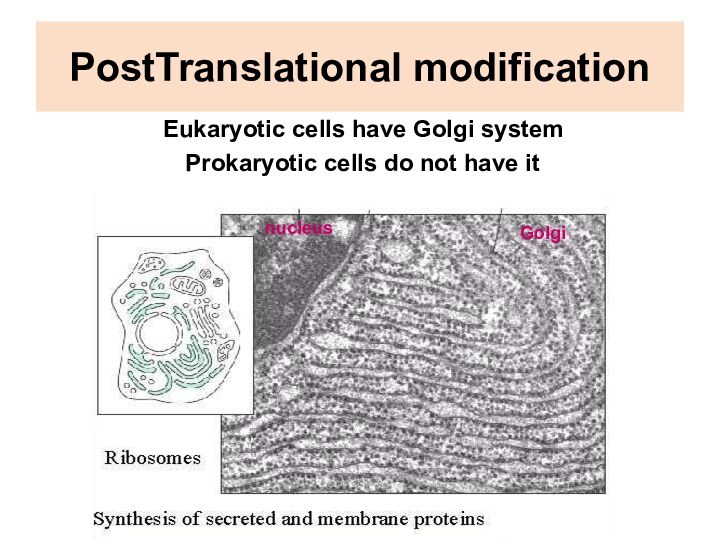
PostTranslational modification
Eukaryotic cells have Golgi system
Prokaryotic
cells do not have it
nucleus
Golgi
Слайд 25
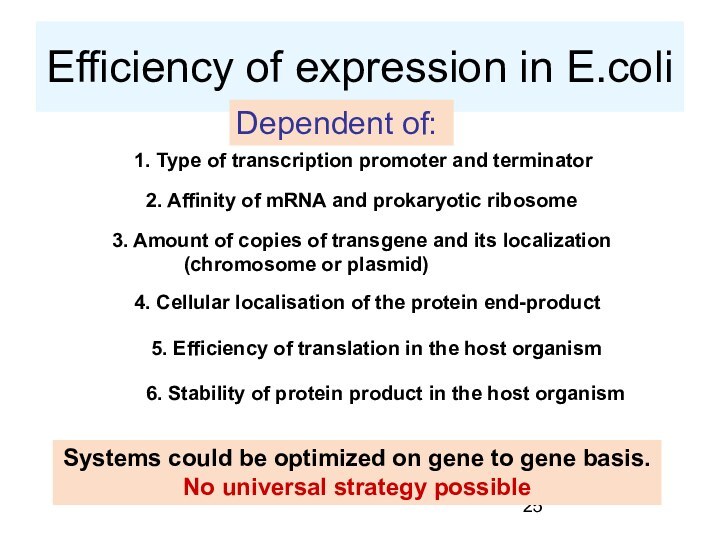
Efficiency of expression in E.coli
Dependent of:
1. Type
of transcription promoter and terminator
2. Affinity of mRNA and
prokaryotic ribosome
3. Amount of copies of transgene and its localization
(chromosome or plasmid)
4. Cellular localisation of the protein end-product
5. Efficiency of translation in the host organism
6. Stability of protein product in the host organism
Systems could be optimized on gene to gene basis.
No universal strategy possible
Слайд 26
Factors affecting transcription
Promoters (including regulated ones)
PROKARYOTIC!!!!
2.
Terminators
PROKARYOTIC!!!!
Слайд 27
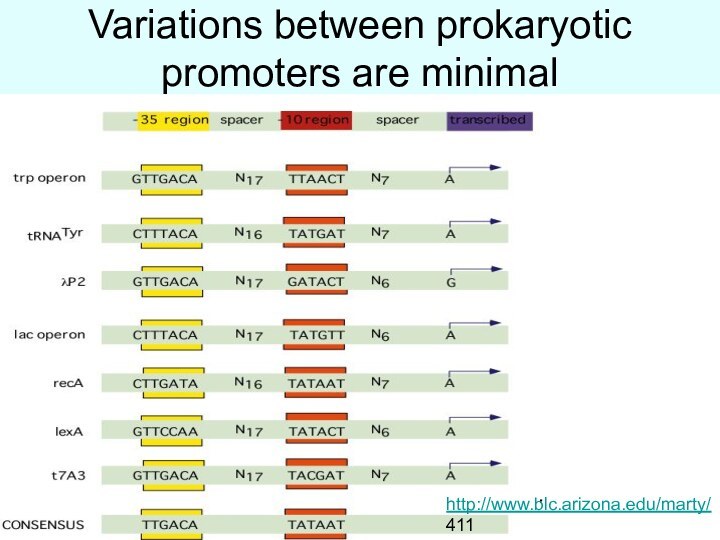
Variations between prokaryotic promoters are minimal
http://www.blc.arizona.edu/marty/
411
Слайд 28
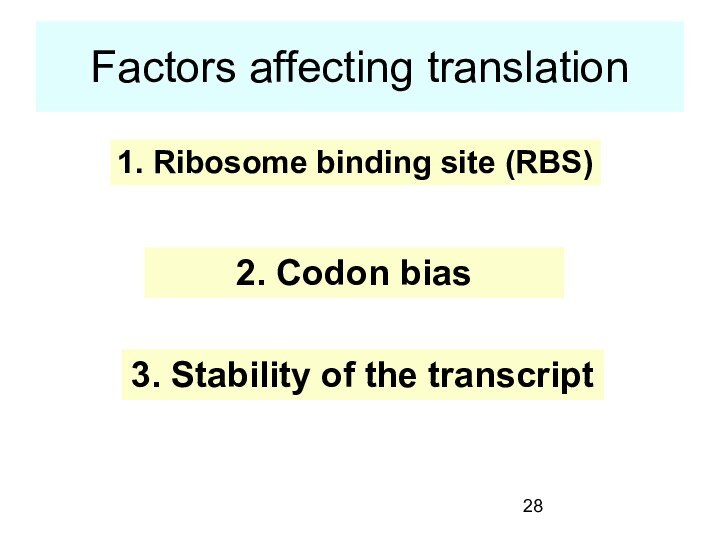
Factors affecting translation
1. Ribosome binding site (RBS)
2. Codon
bias
3. Stability of the transcript
Слайд 29
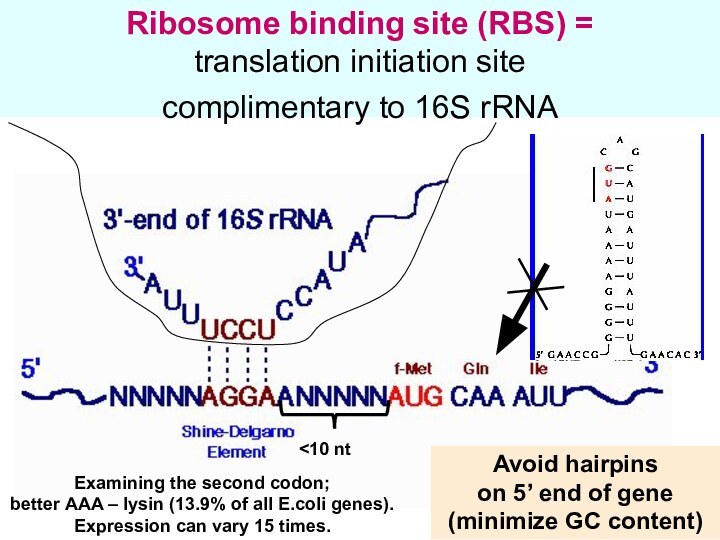
Ribosome binding site (RBS) =
translation initiation site
complimentary to 16S rRNA
end of gene
(minimize GC content)
Examining the second codon;
better AAA – lysin (13.9% of all E.coli genes).
Expression can vary 15 times.
Слайд 30
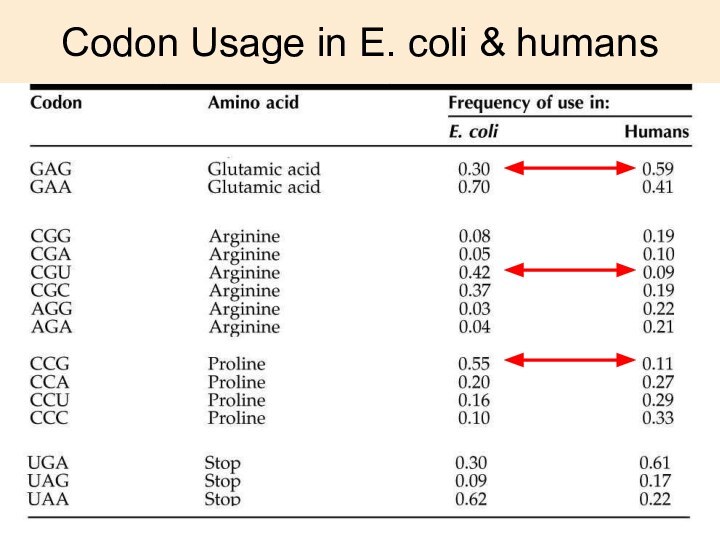
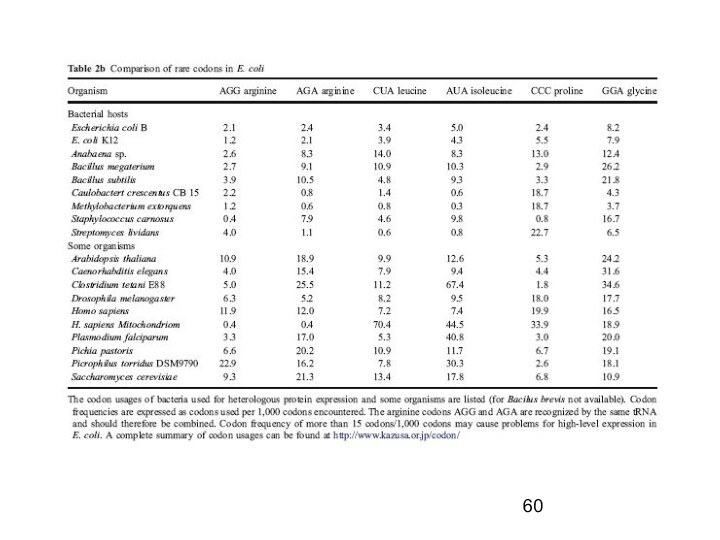
Codon Usage in E. coli & humans
Слайд 31
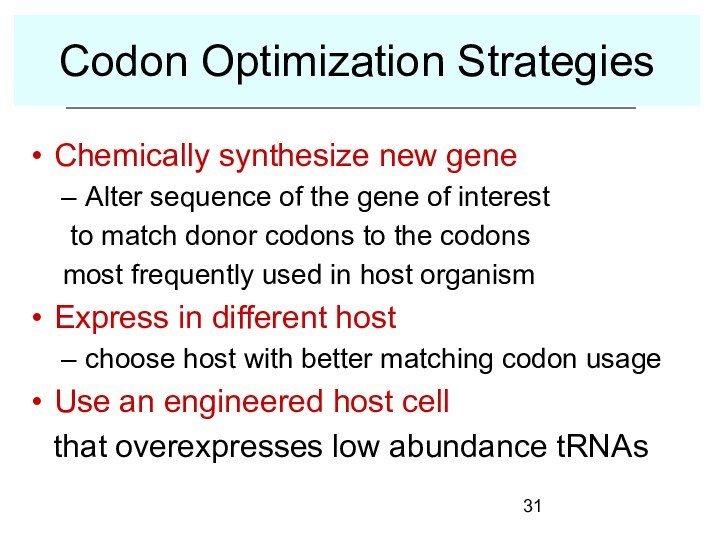
Codon Optimization Strategies
Chemically synthesize new gene
Alter sequence of
the gene of interest
to match donor codons to
the codons
most frequently used in host organism
Express in different host
choose host with better matching codon usage
Use an engineered host cell
that overexpresses low abundance tRNAs
Слайд 32
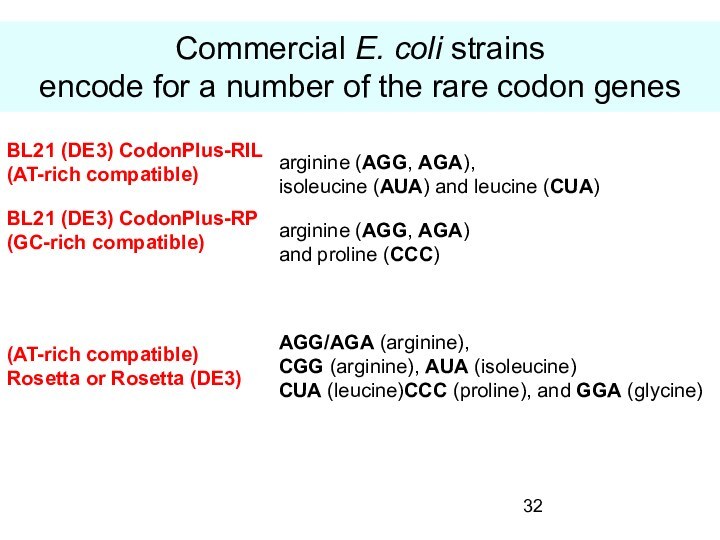
Commercial E. coli strains
encode for a number
of the rare codon genes
Слайд 33
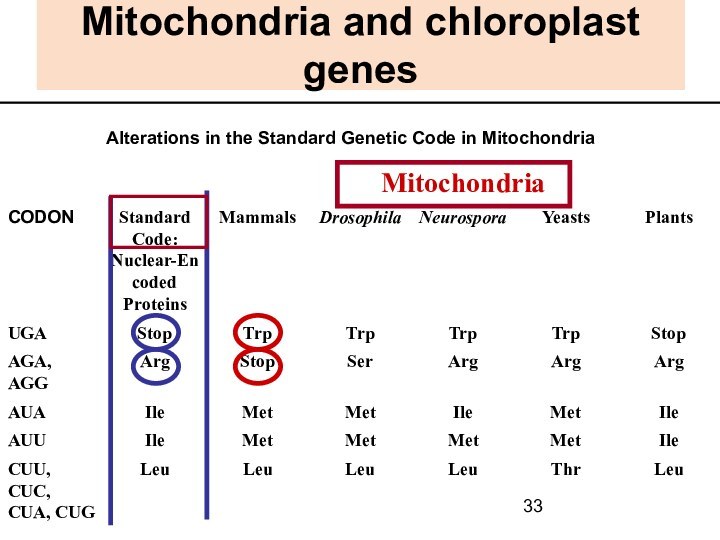
Mitochondria and chloroplast genes
Alterations in the Standard
Genetic Code in Mitochondria
Слайд 34
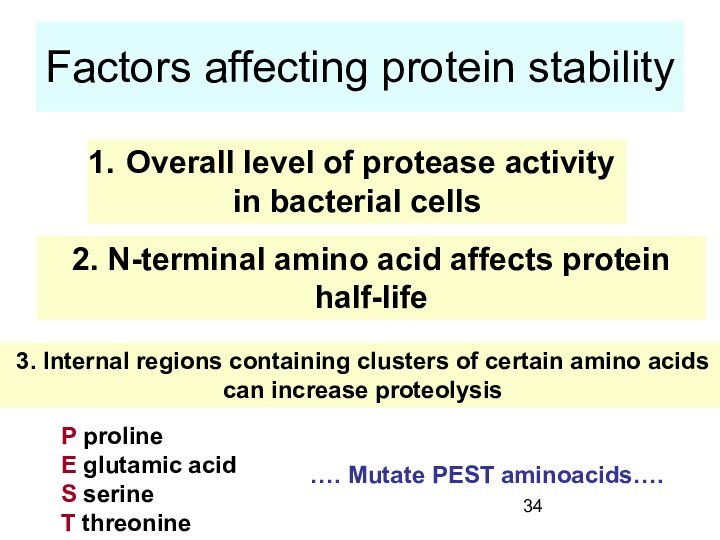
Factors affecting protein stability
Overall level of protease
activity
in bacterial cells
2. N-terminal amino acid affects
protein half-life
3. Internal regions containing clusters of certain amino acids
can increase proteolysis
P proline
E glutamic acid
S serine
T threonine
…. Mutate PEST aminoacids….
Слайд 35
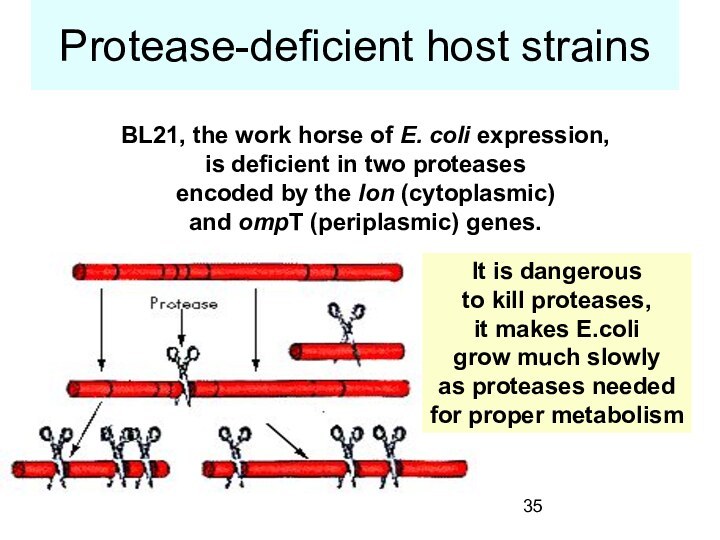
Protease-deficient host strains
BL21, the work horse of
E. coli expression,
is deficient in two proteases
encoded
by the lon (cytoplasmic)
and ompT (periplasmic) genes.
It is dangerous
to kill proteases,
it makes E.coli
grow much slowly
as proteases needed
for proper metabolism
Слайд 36
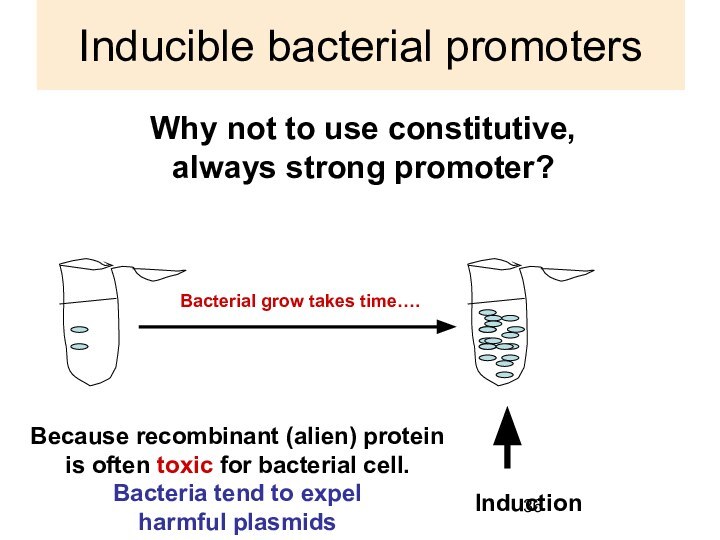
Inducible bacterial promoters
Why not to use constitutive,
always
strong promoter?
Induction
Because recombinant (alien) protein
is often
toxic for bacterial cell.
Bacteria tend to expel
harmful plasmids
Bacterial grow takes time….
Слайд 37
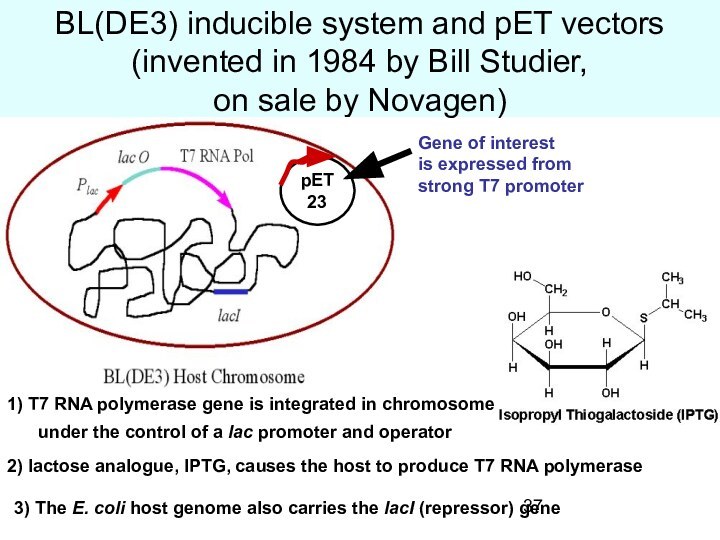
BL(DE3) inducible system and pET vectors (invented in
1984 by Bill Studier,
on sale by Novagen)
1) T7
RNA polymerase gene is integrated in chromosome
under the control of a lac promoter and operator
2) lactose analogue, IPTG, causes the host to produce T7 RNA polymerase
3) The E. coli host genome also carries the lacI (repressor) gene
pET23
Gene of interest
is expressed from
strong T7 promoter
Слайд 38
Why repressor gene and gene of interest are
expressed from different DNA molecules?
Repressor gene expressed from
chromosome;
Gene of Interest expressed from plasmid
If too high repressor ? no transcription
(you need to increase expensive IPTG)
If too low repressor ? promoter is leaky
(active without IPTG)
Repressor is in chromosome,
because there it is best kept controlled there
(no plasmid loss, not too high expression)
Слайд 39
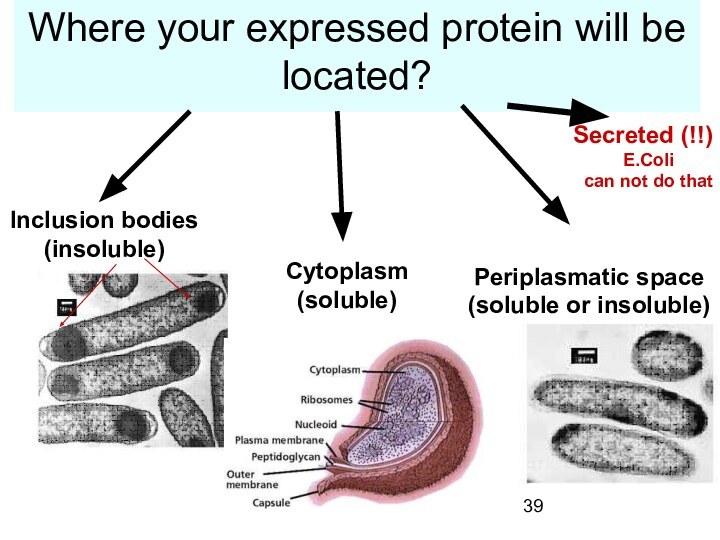
Where your expressed protein will be located?
Inclusion
bodies
(insoluble)
Cytoplasm
(soluble)
Periplasmatic space
(soluble or insoluble)
Secreted (!!)
E.Coli
can
not do that
Слайд 40
1. Inclusion bodies
(most common case)
-- Inclusion
bodies are formed through the accumulation
of folding intermediates
rather than from the native or unfolded proteins.
-- It is not possible to predict which proteins
will be produced as inclusion bodies.
-- Production of inclusion bodies
not dependent on the origin of protein,
the used promoters,
the hydrophobicity of target proteins...
Слайд 41
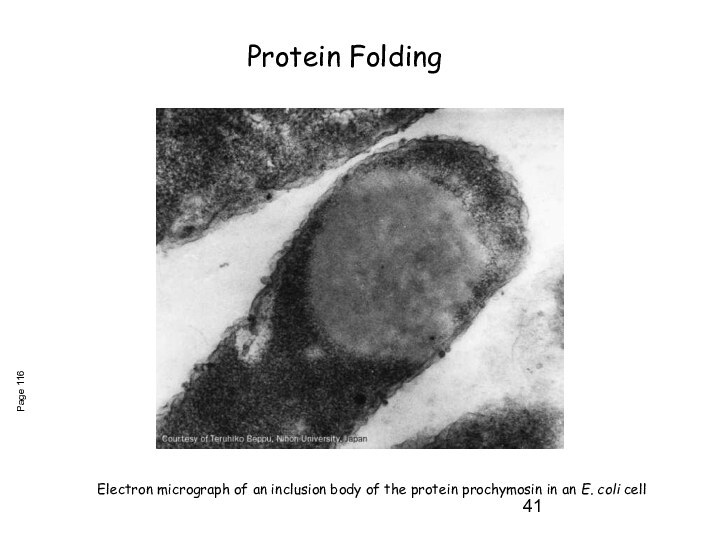
Electron micrograph of an inclusion body of the
protein prochymosin in an E. coli cell
Page 116
Protein Folding
Слайд 42
Good side of inclusion bodies
inclusion bodies can be
accumulated in the cytoplasm
to much higher level (greater
than 25%)
than production as soluble form;
2) inclusion bodies is initially isolated
in a highly purified, solid, and concentrated state
by simple physical operation (centrifugation).
3) inclusion bodies have no biological activity.
For toxic proteins it may be the only one available;
4) inclusion bodies are resistant to proteolysis
That results in the high yield of protein production.
Слайд 43
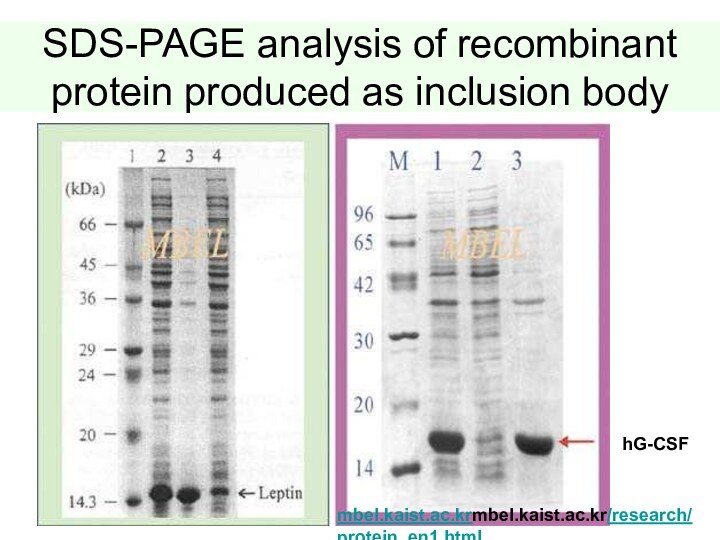
SDS-PAGE analysis of recombinant protein produced as inclusion
body
hG-CSF
mbel.kaist.ac.krmbel.kaist.ac.kr/research/ protein_en1.html
Слайд 44
Recovery of proteins from inclusion bodies
Is not a
straightforward process, but road of trials and errors
Solubilization
Refolding
Choice of
solubilizing agents,
e.g., urea,
guanidine HCl,
or detergents,
plays a key role
in solubilization
efficiency
-- Refolding is initiated
by reducing concentration
of denaturant used to solubilize IBs.
Guandinium
-- Refolding competes with other reactions,
such as misfolding and aggregation
(both are leading to bad results)
-- Chaperones are helpful in refolding
(including chemical chaperones)
Слайд 45
Question of questions –
how to purify your
protein?
Слайд 46
Diversity of proteins could be exploited
Column chromatography
Matrix
particles
usually packed in the column
in the form
of small beads.
A protein purification strategy
might employ in turn each of the three kinds of matrix
described below,
with a final protein purification
Of up to 10,000-fold.
Essential Cell Biology:
An Introduction to the Molecular Biology of the Cell
Слайд 47
Column chromatography
Different proteins
are retarded to different extents
by their interaction with the matrix,
they can be
collected separately
as they flow out from the bottom.
According to the choice of matrix,
proteins can be separated
according to
-- their charge,
-- their hydrophobicity,
-- their size,
-- their ability to bind to
particular chemical groups (!!)
Essential Cell Biology:
An Introduction to the Molecular Biology of the Cell
Слайд 48
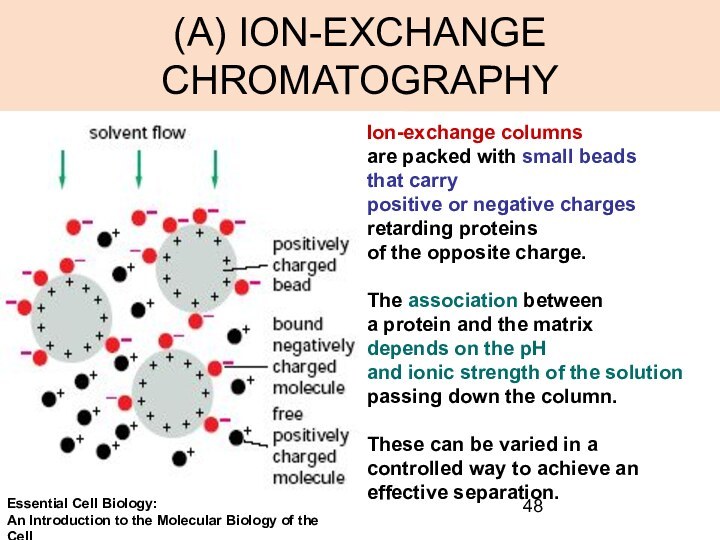
(A) ION-EXCHANGE CHROMATOGRAPHY
Ion-exchange columns
are packed with small
beads
that carry
positive or negative charges
retarding proteins
of
the opposite charge.
The association between
a protein and the matrix
depends on the pH
and ionic strength of the solution
passing down the column.
These can be varied in a
controlled way to achieve an effective separation.
Essential Cell Biology:
An Introduction to the Molecular Biology of the Cell
Слайд 49
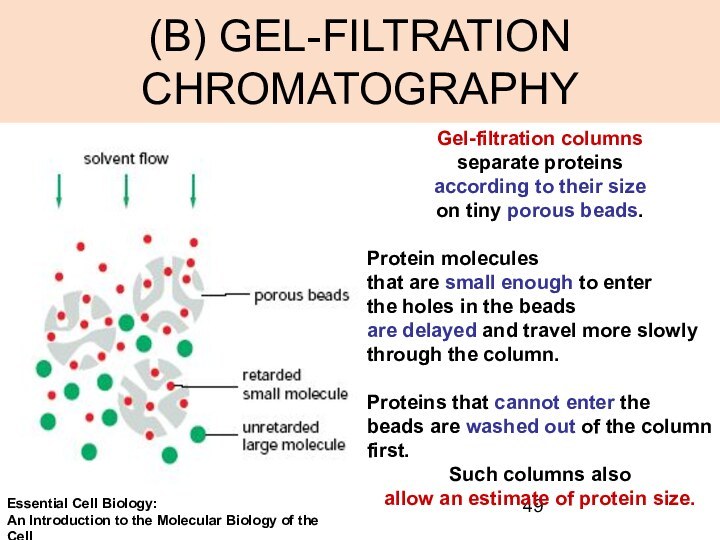
(B) GEL-FILTRATION CHROMATOGRAPHY
Gel-filtration columns
separate proteins
according to
their size
on tiny porous beads.
Protein molecules
that are
small enough to enter
the holes in the beads
are delayed and travel more slowly
through the column.
Proteins that cannot enter the beads are washed out of the column first.
Such columns also
allow an estimate of protein size.
Essential Cell Biology:
An Introduction to the Molecular Biology of the Cell
Слайд 50
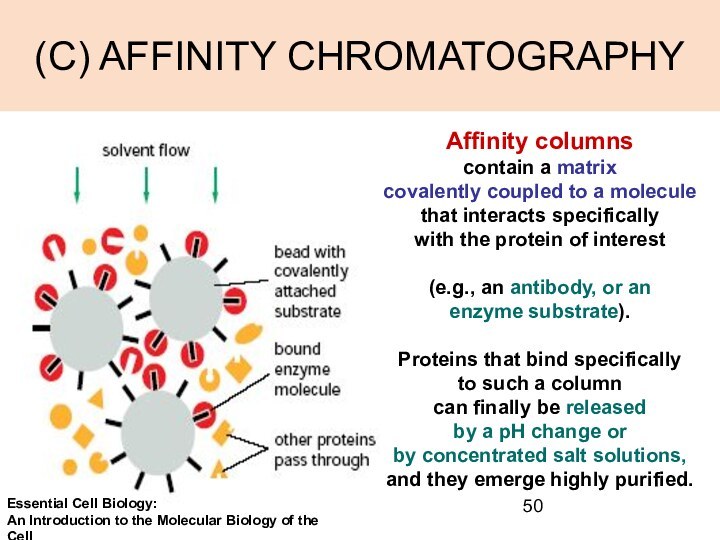
(C) AFFINITY CHROMATOGRAPHY
Affinity columns
contain a matrix
covalently coupled
to a molecule that interacts specifically
with the protein
of interest
(e.g., an antibody, or an
enzyme substrate).
Proteins that bind specifically
to such a column
can finally be released
by a pH change or
by concentrated salt solutions,
and they emerge highly purified.
Essential Cell Biology:
An Introduction to the Molecular Biology of the Cell
Слайд 51
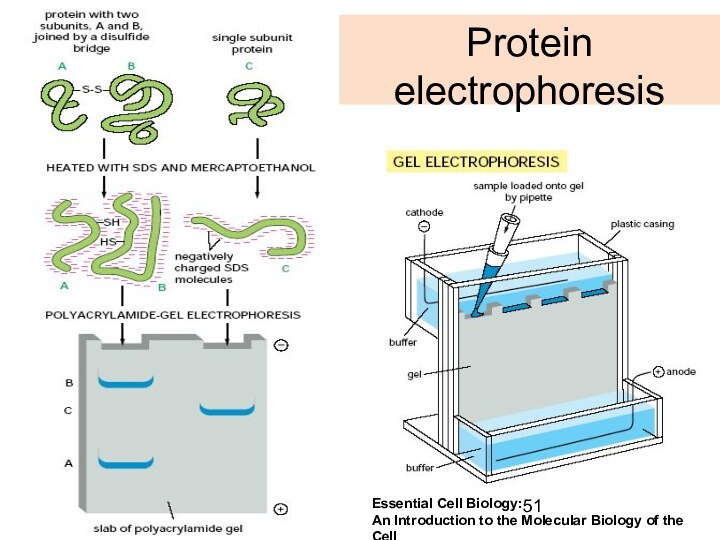
Protein
electrophoresis
Essential Cell Biology:
An Introduction to
the Molecular Biology of the Cell
Слайд 52
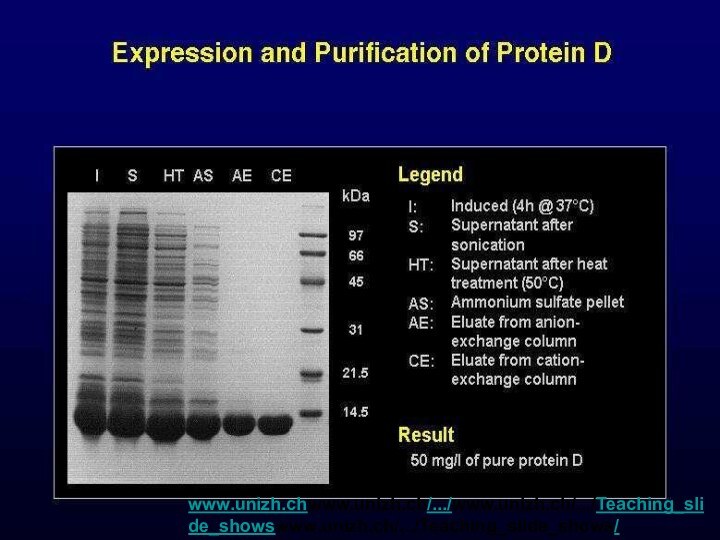
www.unizh.chwww.unizh.ch/.../www.unizh.ch/.../Teaching_slide_showswww.unizh.ch/.../Teaching_slide_shows/ Lambda/sld015.htm
www.unizh.chwww.unizh.ch/.../www.unizh.ch/.../Teaching_slide_showswww.unizh.ch/.../Teaching_slide_shows/ Lambda/sld015.htm
Слайд 53
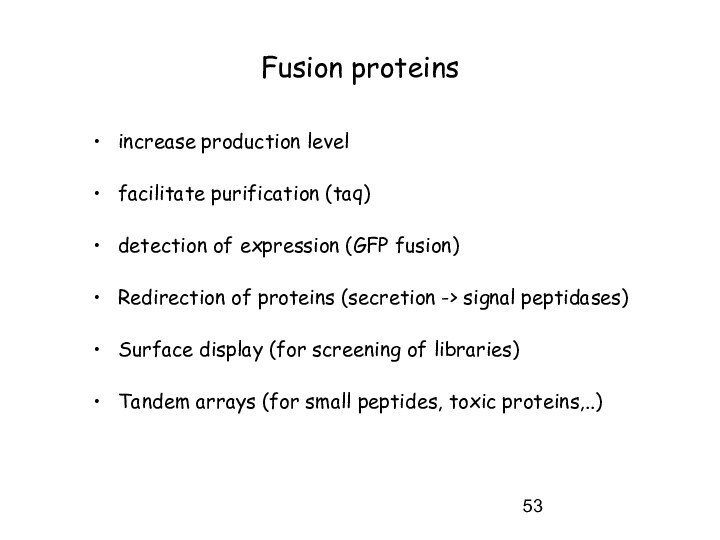
Fusion proteins
increase production level
facilitate purification (taq)
detection of expression
(GFP fusion)
Redirection of proteins (secretion -> signal peptidases)
Surface display
(for screening of libraries)
Tandem arrays (for small peptides, toxic proteins,..)
Слайд 54
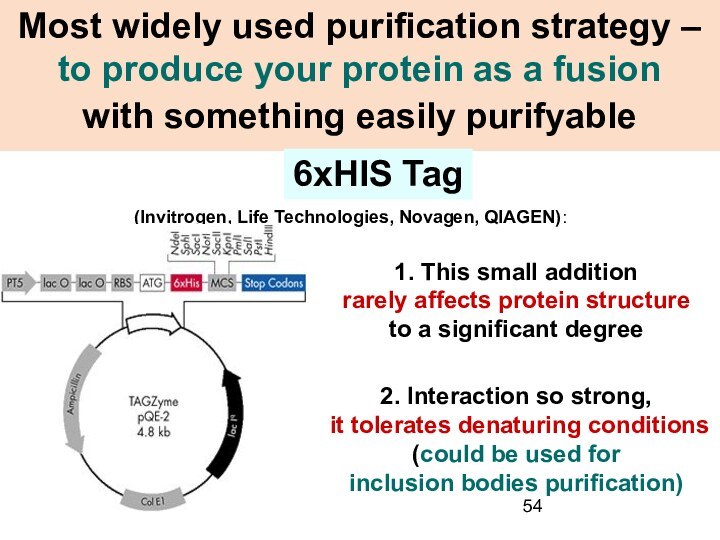
Most widely used purification strategy – to produce
your protein as a fusion
with something easily purifyable
(Invitrogen, Life Technologies, Novagen, QIAGEN):
6xHIS Tag
1. This small addition
rarely affects protein structure
to a significant degree
2. Interaction so strong,
it tolerates denaturing conditions
(could be used for
inclusion bodies purification)
Слайд 55
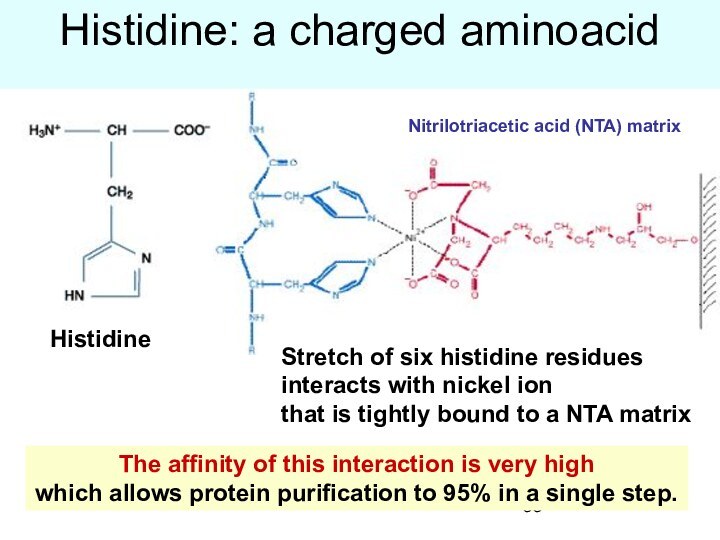
Histidine: a charged aminoacid
The affinity of this
interaction is very high
which allows protein purification to
95% in a single step.
Stretch of six histidine residues
interacts with nickel ion
that is tightly bound to a NTA matrix
Nitrilotriacetic acid (NTA) matrix
Histidine
Слайд 56
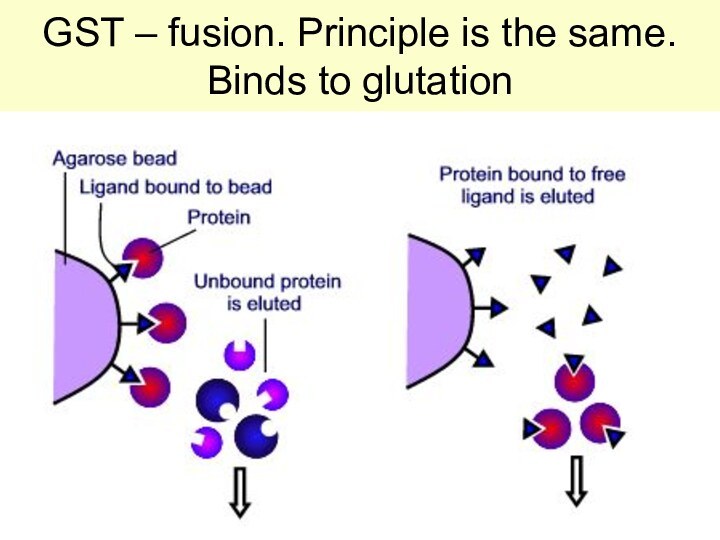
GST – fusion. Principle is the same. Binds
to glutation
Слайд 57
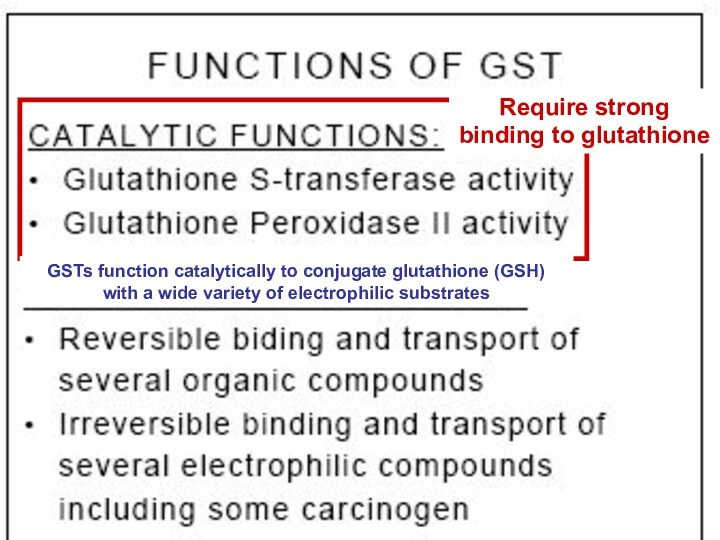
Require strong
binding to glutathione
Require strong
binding
to glutathione
GSTs function catalytically to conjugate glutathione (GSH)
with a wide variety of electrophilic substrates
Слайд 58
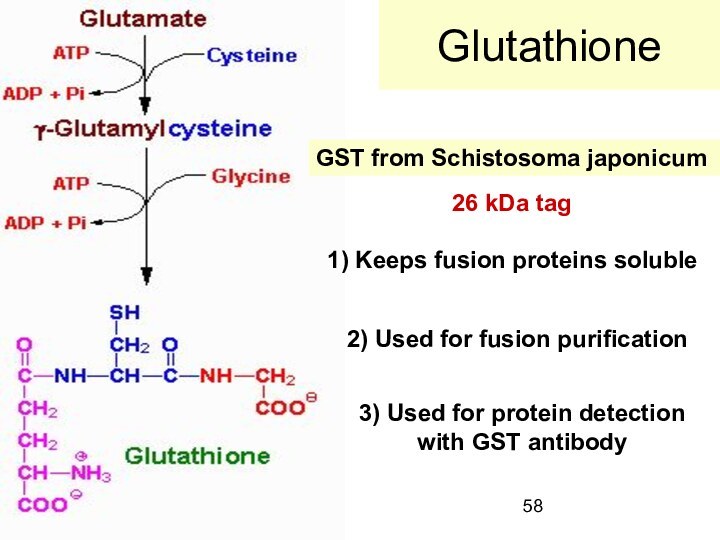
Glutathione
GST from Schistosoma japonicum
1) Keeps fusion proteins
soluble
2) Used for fusion purification
3) Used for protein
detection
with GST antibody
26 kDa tag
Слайд 59
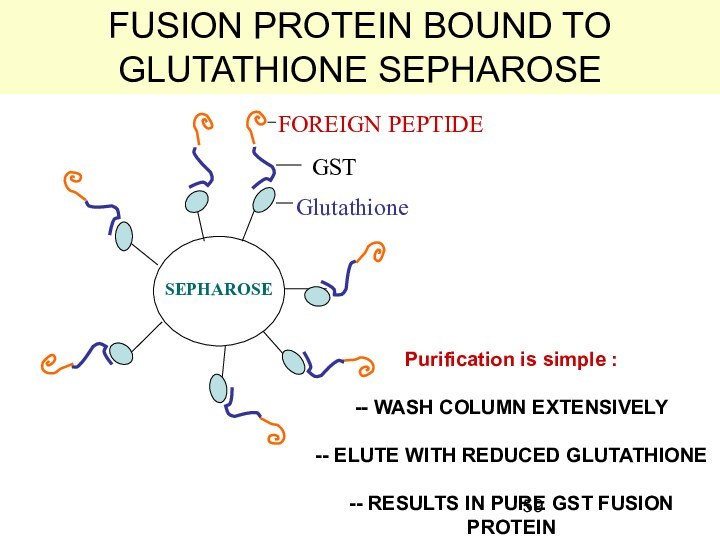
FUSION PROTEIN BOUND TO GLUTATHIONE SEPHAROSE
Glutathione
GST
FOREIGN PEPTIDE
SEPHAROSE
Purification is
simple :
-- WASH COLUMN EXTENSIVELY
-- ELUTE WITH REDUCED
GLUTATHIONE
-- RESULTS IN PURE GST FUSION PROTEIN
Слайд 61
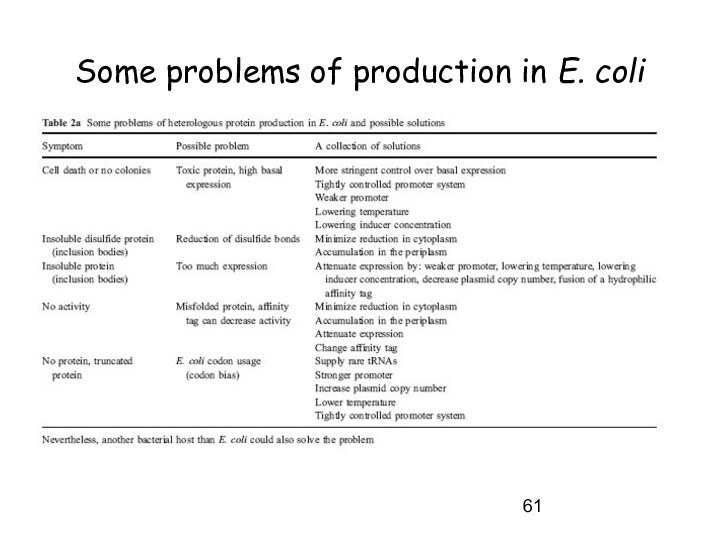
Some problems of production in E. coli
Слайд 62
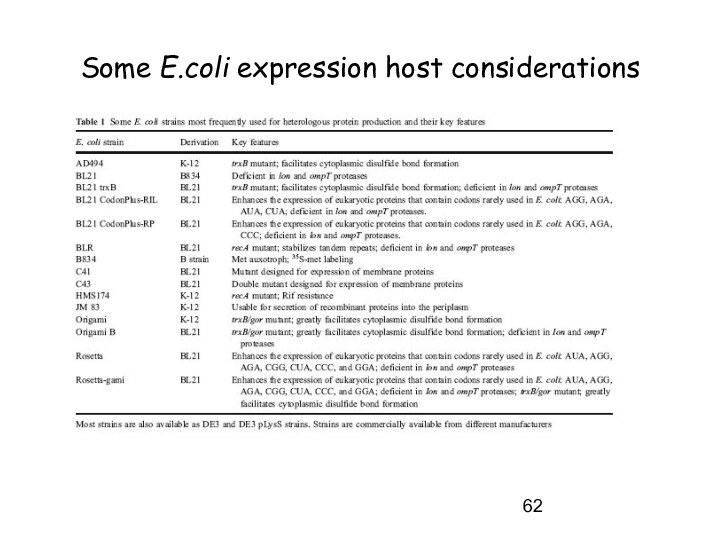
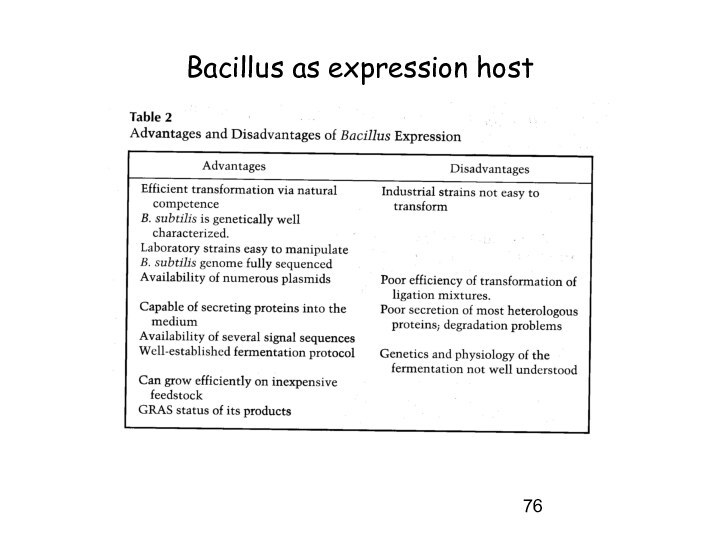
Some E.coli expression host considerations
Слайд 63
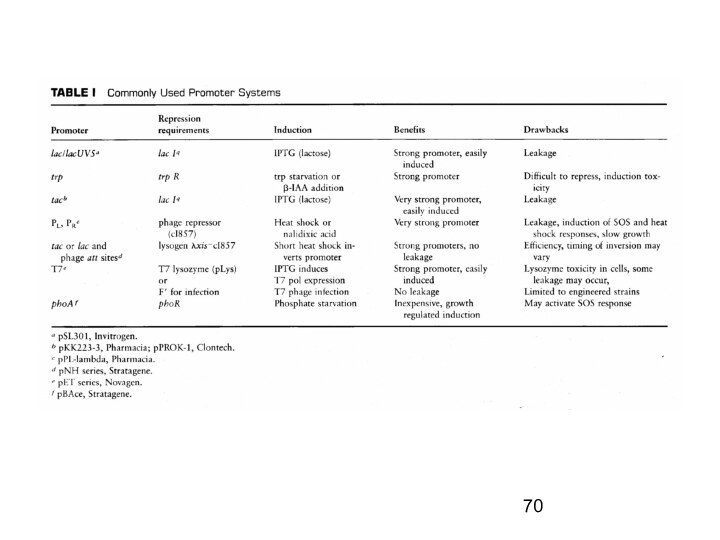
Principal factors in bacterial expression
Слайд 65
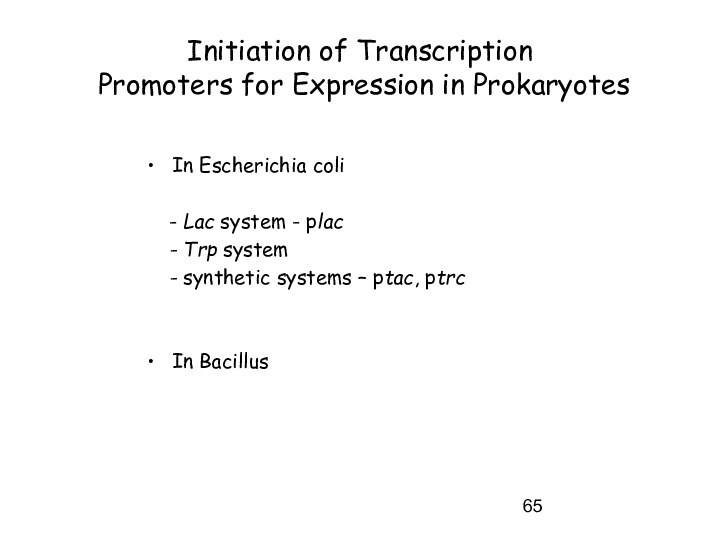
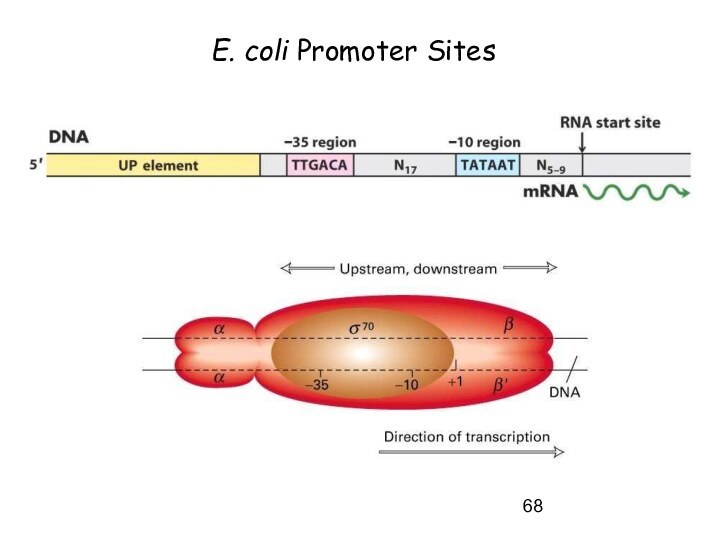
Initiation of Transcription
Promoters for Expression in
Prokaryotes
In Escherichia coli
- Lac system - plac
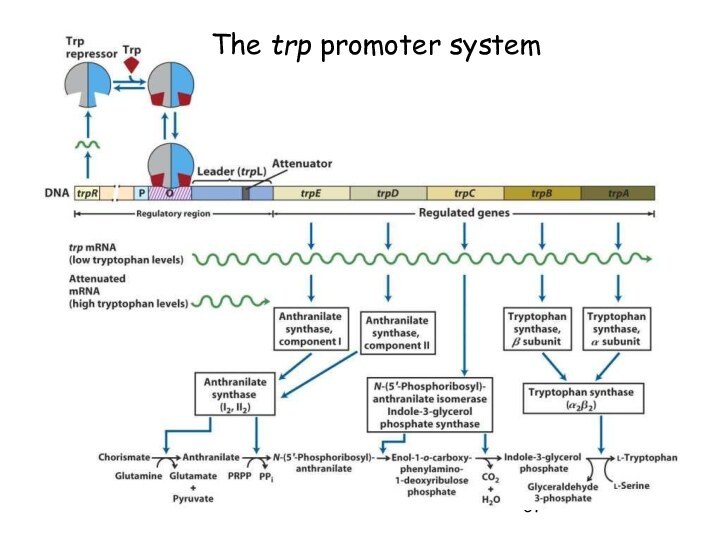
- Trp system
- synthetic systems – ptac, ptrc
In Bacillus
Слайд 69
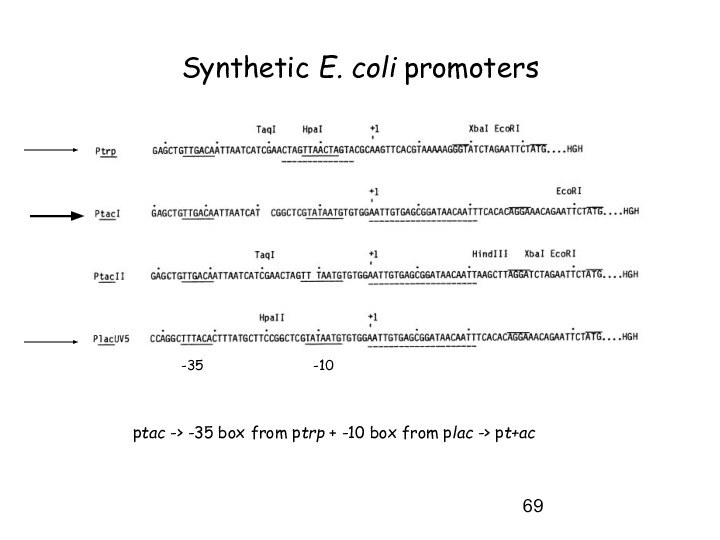
Synthetic E. coli promoters
-35
-10
ptac -> -35 box from
ptrp + -10 box from plac -> pt+ac
Слайд 71
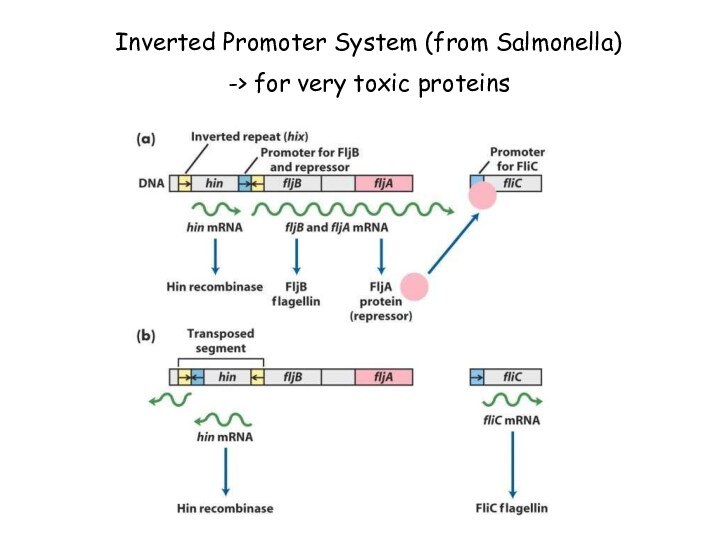
Inverted Promoter System (from Salmonella)
-> for very
toxic proteins
Слайд 72
Bacillus
In 1872, Ferdinand Cohn, a student of Robert
Koch, recognized and named the bacterium Bacillus subtilis.
The
organism was made to represent a large and diverse genus of Bacteria, Bacillus, and was placed in the family Bacillaceae.
The family's distinguishing feature is the production of endospores, which are highly refractile resting structures formed within the bacterial cells. Since this time, members of the genus Bacillus are characterized as Gram-positive, rod-shaped, aerobic or facultative, endospore-forming bacteria.
Flagellar stains of various species of Bacillus from CDC
Слайд 73
Bacillus
Antibiotic Producers: B. brevis (e.g. gramicidin, tyrothricin), B.
cereus (e.g. cerexin, zwittermicin), B. circulans (e.g. circulin), B.
laterosporus (e.g. laterosporin), B. licheniformis (e.g. bacitracin), B. polymyxa (e.g. polymyxin, colistin), B. pumilus (e.g. pumulin) B. subtilis (e.g. polymyxin, difficidin, subtilin, mycobacillin).
Pathogens of Insects: B. larvae, B. lentimorbis, and B. popilliae are invasive pathogens. B. thuringiensis forms a parasporal crystal that is toxic to beetles.
Pathogens of Animals: B. anthracis, and B. cereus. B. alvei, B. megaterium, B. coagulans, B. laterosporus, B. subtilis, B. sphaericus, B. circulans, B. brevis, B. licheniformis, B. macerans, B. pumilus, and B. thuringiensis have been isolated from human infections.
The Genus Bacillus includes two bacteria of significant medical importance, B. anthracis, the causative agent of anthrax, and B. cereus, which causes food poisoning. Nonanthrax Bacillus species can also cause a wide variety of other infections, and they are being recognized with increasing frequency as pathogens in humans.
Слайд 74
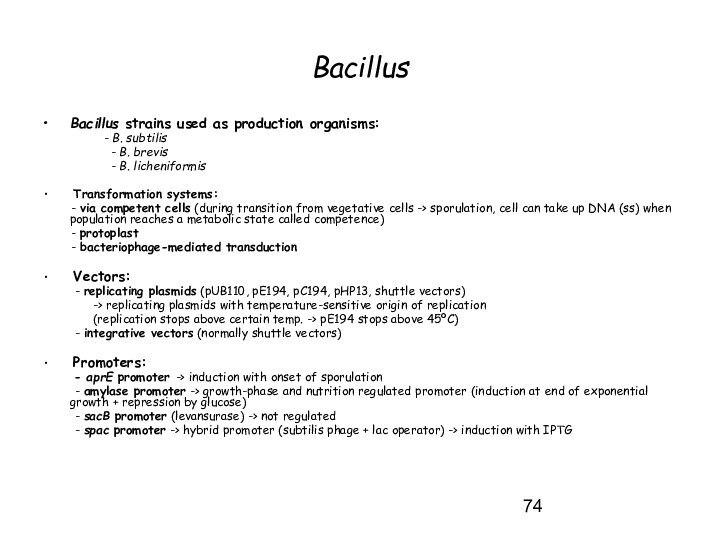
Bacillus
Bacillus strains used as production organisms:
- B. subtilis
- B. brevis
- B. licheniformis
Transformation systems:
- via competent cells (during transition from vegetative cells -> sporulation, cell can take up DNA (ss) when population reaches a metabolic state called competence)
- protoplast
- bacteriophage-mediated transduction
Vectors:
- replicating plasmids (pUB110, pE194, pC194, pHP13, shuttle vectors)
-> replicating plasmids with temperature-sensitive origin of replication
(replication stops above certain temp. -> pE194 stops above 45ºC)
- integrative vectors (normally shuttle vectors)
Promoters:
- aprE promoter -> induction with onset of sporulation
- amylase promoter -> growth-phase and nutrition regulated promoter (induction at end of exponential growth + repression by glucose)
- sacB promoter (levansurase) -> not regulated
- spac promoter -> hybrid promoter (subtilis phage + lac operator) -> induction with IPTG
Слайд 77
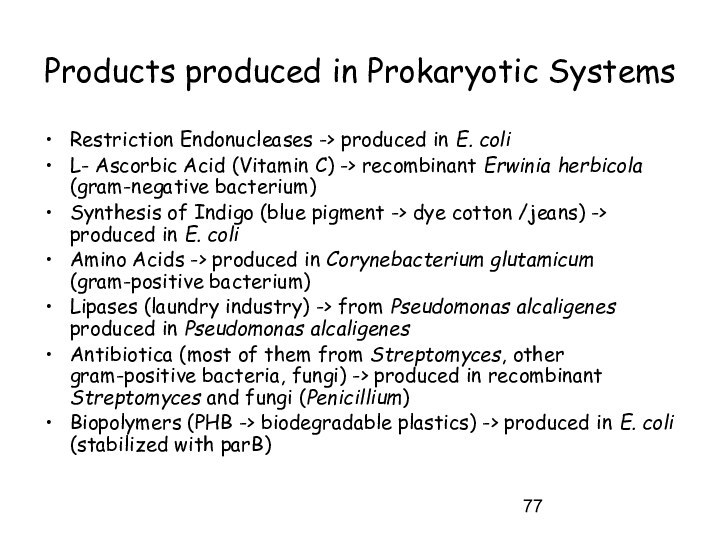
Products produced in Prokaryotic Systems
Restriction Endonucleases ->
produced in E. coli
L- Ascorbic Acid (Vitamin C) ->
recombinant Erwinia herbicola (gram-negative bacterium)
Synthesis of Indigo (blue pigment -> dye cotton /jeans) -> produced in E. coli
Amino Acids -> produced in Corynebacterium glutamicum (gram-positive bacterium)
Lipases (laundry industry) -> from Pseudomonas alcaligenes produced in Pseudomonas alcaligenes
Antibiotica (most of them from Streptomyces, other gram-positive bacteria, fungi) -> produced in recombinant Streptomyces and fungi (Penicillium)
Biopolymers (PHB -> biodegradable plastics) -> produced in E. coli (stabilized with parB)
Слайд 78
Expression in Eukaryotic Systems
Yeast
- Saccharomyces cerevisiae
(baker’s yeast)
- Pichia pastoris
Insect Cells – Baculovirus
Mammalian Cells
Слайд 79
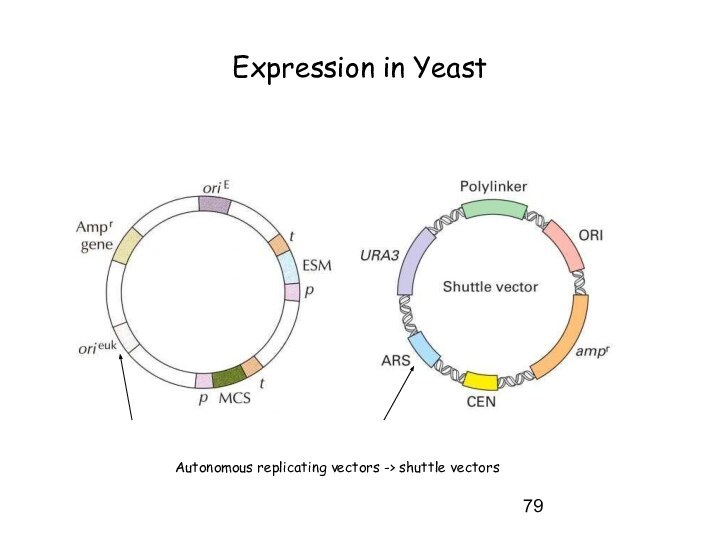
Expression in Yeast
Autonomous replicating vectors -> shuttle vectors
Слайд 80
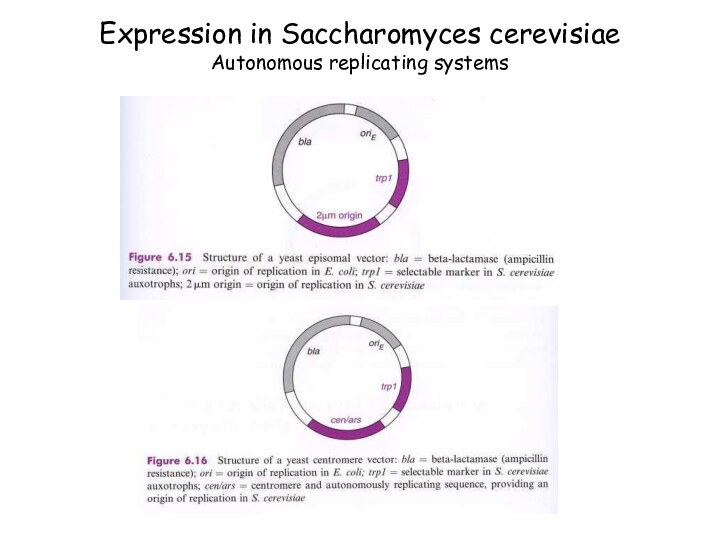
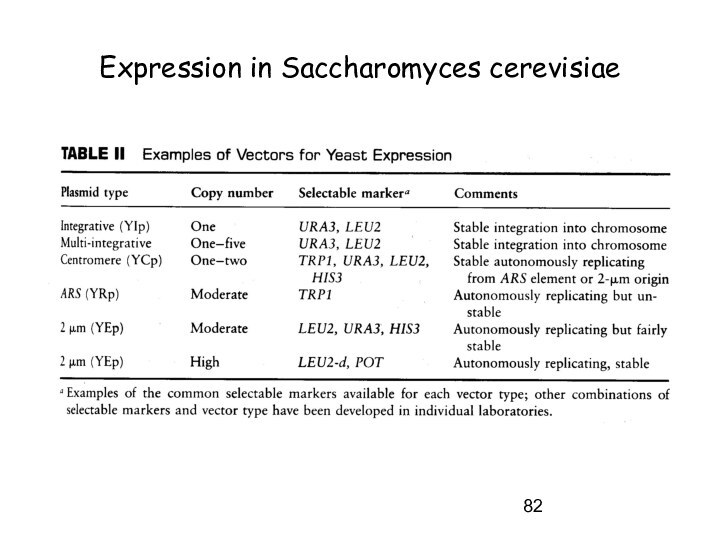
Expression in Saccharomyces cerevisiae
Autonomous replicating systems
Слайд 81
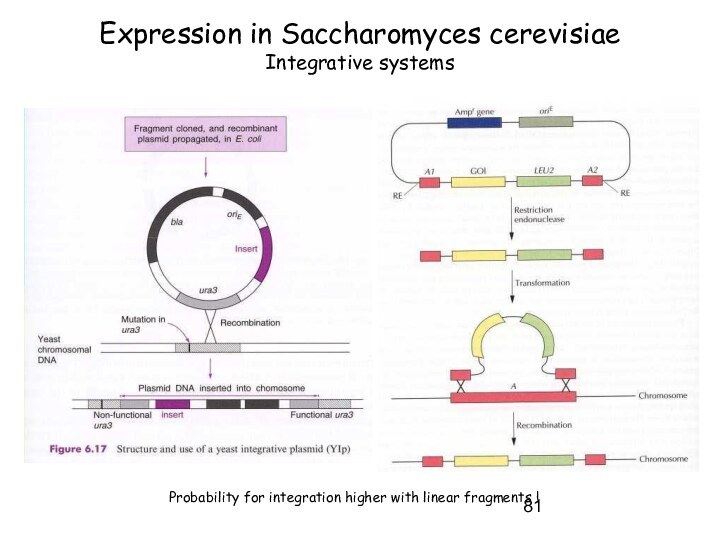
Expression in Saccharomyces cerevisiae
Integrative systems
Probability for integration higher
with linear fragments !
Слайд 82
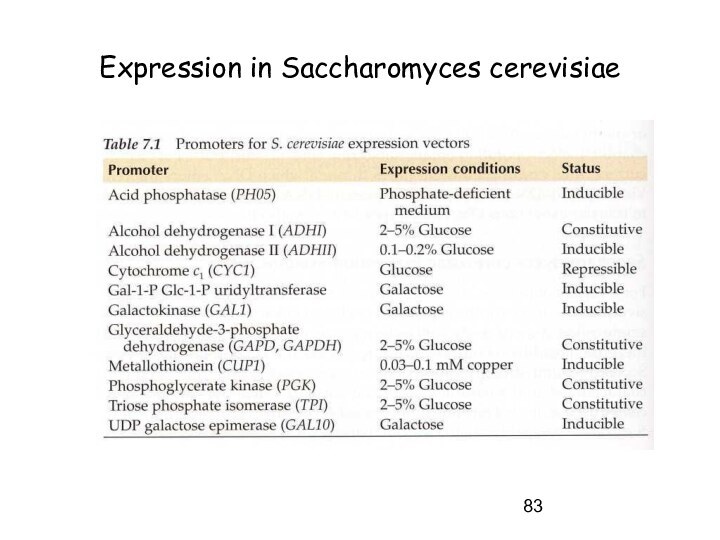
Expression in Saccharomyces cerevisiae
Слайд 83
Expression in Saccharomyces cerevisiae
Слайд 84
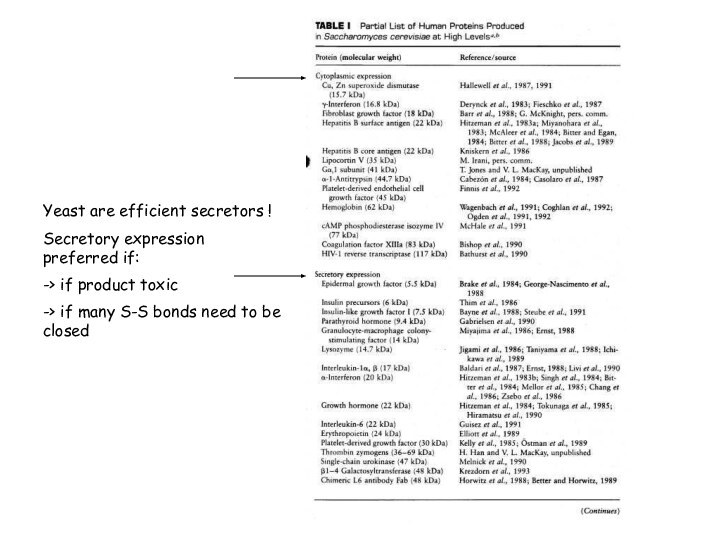
Yeast are efficient secretors !
Secretory expression preferred if:
->
if product toxic
-> if many S-S bonds need to
be closed
Слайд 85
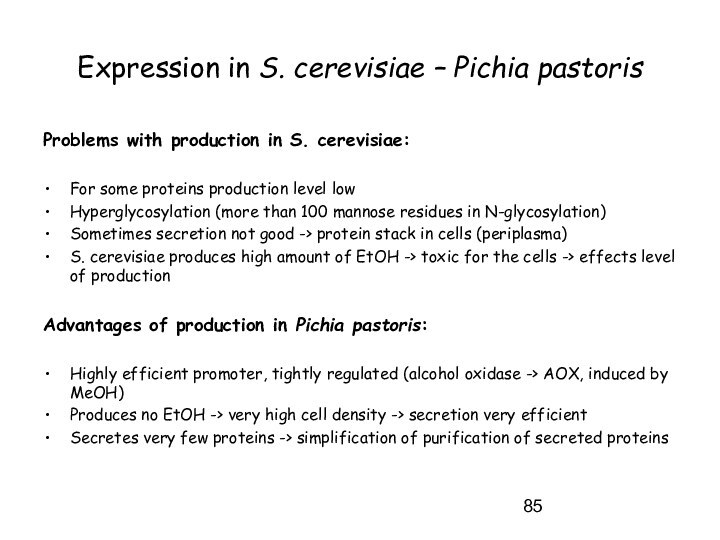
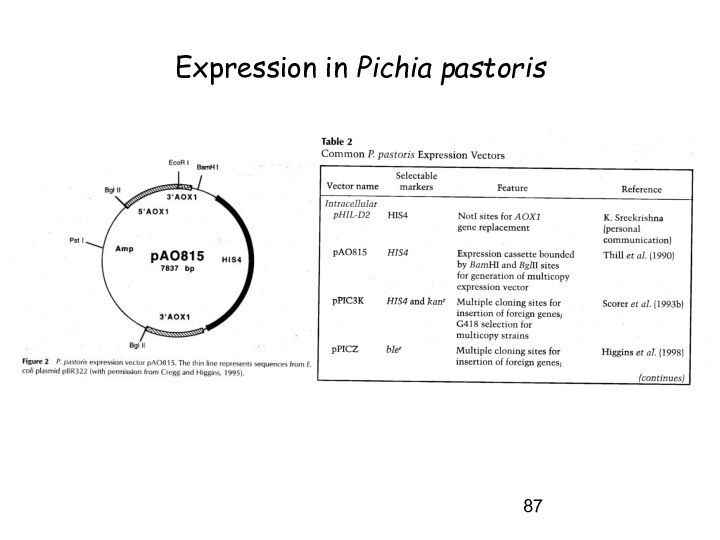
Expression in S. cerevisiae – Pichia pastoris
Problems with
production in S. cerevisiae:
For some proteins production level low
Hyperglycosylation
(more than 100 mannose residues in N-glycosylation)
Sometimes secretion not good -> protein stack in cells (periplasma)
S. cerevisiae produces high amount of EtOH -> toxic for the cells -> effects level of production
Advantages of production in Pichia pastoris:
Highly efficient promoter, tightly regulated (alcohol oxidase -> AOX, induced by MeOH)
Produces no EtOH -> very high cell density -> secretion very efficient
Secretes very few proteins -> simplification of purification of secreted proteins
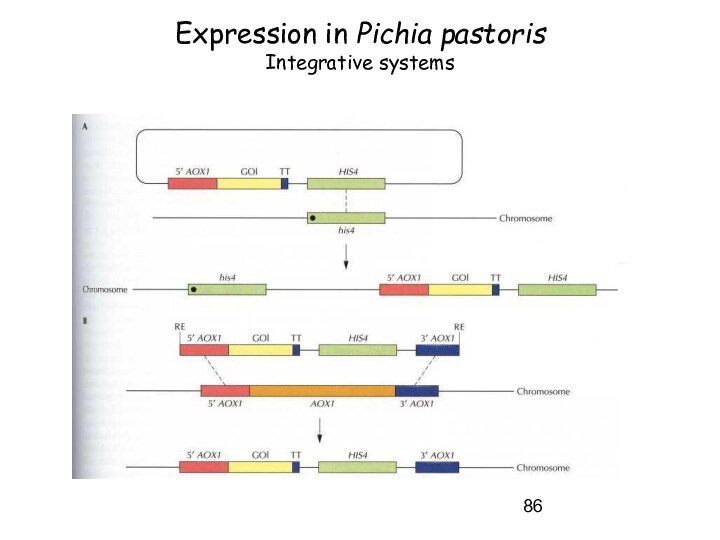
Слайд 86
Expression in Pichia pastoris
Integrative systems
Слайд 89
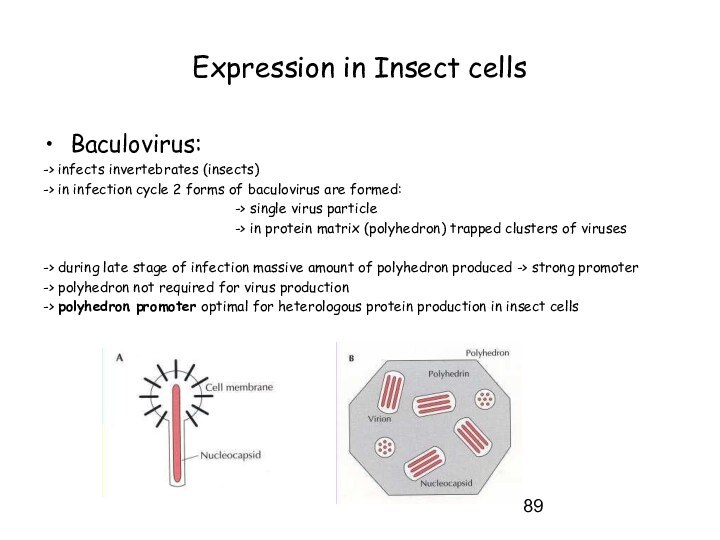
Expression in Insect cells
Baculovirus:
-> infects invertebrates (insects)
-> in
infection cycle 2 forms of baculovirus are formed:
-> single virus particle
-> in protein matrix (polyhedron) trapped clusters of viruses
-> during late stage of infection massive amount of polyhedron produced -> strong promoter
-> polyhedron not required for virus production
-> polyhedron promoter optimal for heterologous protein production in insect cells
Слайд 90
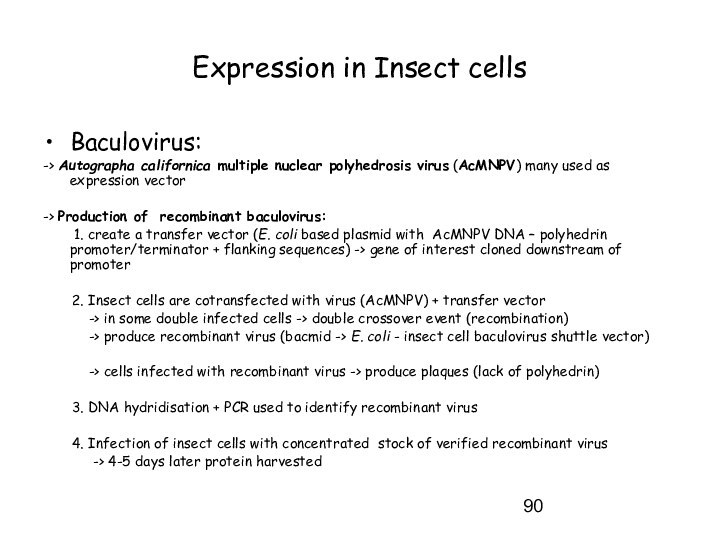
Expression in Insect cells
Baculovirus:
-> Autographa californica multiple nuclear
polyhedrosis virus (AcMNPV) many used as expression vector
-> Production
of recombinant baculovirus:
1. create a transfer vector (E. coli based plasmid with AcMNPV DNA – polyhedrin promoter/terminator + flanking sequences) -> gene of interest cloned downstream of promoter
2. Insect cells are cotransfected with virus (AcMNPV) + transfer vector
-> in some double infected cells -> double crossover event (recombination)
-> produce recombinant virus (bacmid -> E. coli - insect cell baculovirus shuttle vector)
-> cells infected with recombinant virus -> produce plaques (lack of polyhedrin)
3. DNA hydridisation + PCR used to identify recombinant virus
4. Infection of insect cells with concentrated stock of verified recombinant virus
-> 4-5 days later protein harvested
Слайд 92
Why this system?
Insect cells have almost the same
posttranslational modifications as mammalian cells
Higher expression level than mammalian
cells
Baculovirus expression system
Слайд 93
Mammalian cell expression system
1. Why do we use
that system?
-> to get full complement of
posttranslational modifications on proteins
2. Developed cell lines:
-> short term (transient) expression -> autonomous replicating systems -> viral origins (SV40)
- African green monkey kidney (COS)
- baby hamster kidney (BHK)
- human embryonic kidney (HEK-239)
-> long term (stable) expression -> integration into chromosome -> viral origins
- chinese hamster ovary (CHO)
Слайд 94
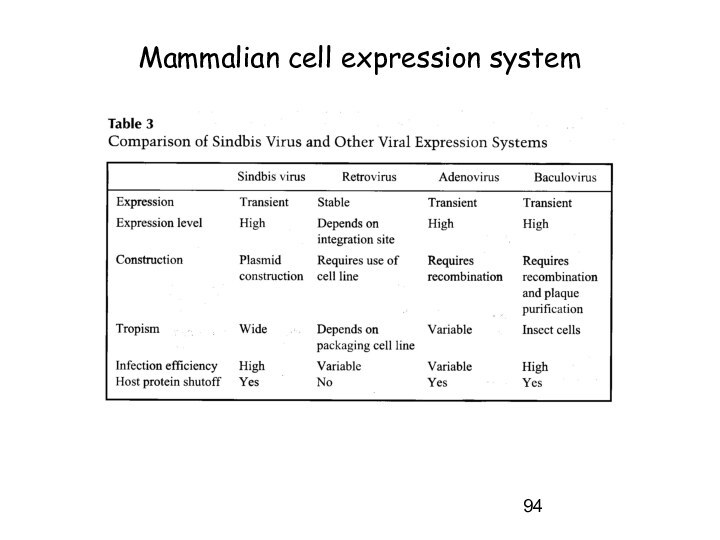
Mammalian cell expression system
Слайд 95
Gene expression in mammalian cell lines
A convenient alternative

for setting up mammalian cell facilities – get a
comprehensive service from us. We will achieve stable expression of the gene of your interest in mammalian cells.
Customer provides:
- Mammalian vector with the gene (cDNA) to be expressed. We accept plasmid and retroviral vectors
- Sequence of the gene and map of the construct for transfection
Cell line or information about the cell line to be transfected.
Our service includes:
- Transfection of the cells. In case of a retroviral vector, virus production and cell infection
- Antibiotic selection and generation of stable transfected (infected) cell clones. At least 10 independent clones will be selected and grown
- Quantitative assay of the gene (cDNA) expression level in each transfected clone by RNA isolation followed by Northern hybridisation and/or RT-PCR
- Selection of the best expressing clone
- Cell freezing and depositing
- Duration: 3-6 months (depending on the cell growth rate), allow 1month in addition if the cell line is not available in our collections
Customer receives:
- Detailed report on experiments and data obtained.
- Two vials of transfected cells (the best expressing clone)
- We will deposit the transfected cells in our collection as a precaution against accidental loss of the clone.
Price guide:
Price per transfection and selection of at least 10 clones: £3500.