- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
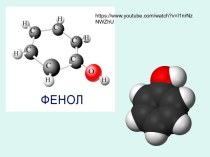
Презентация на тему Types of chemical bonds in crystals
Содержание
- 2. Atoms bond during chemical reactions to result
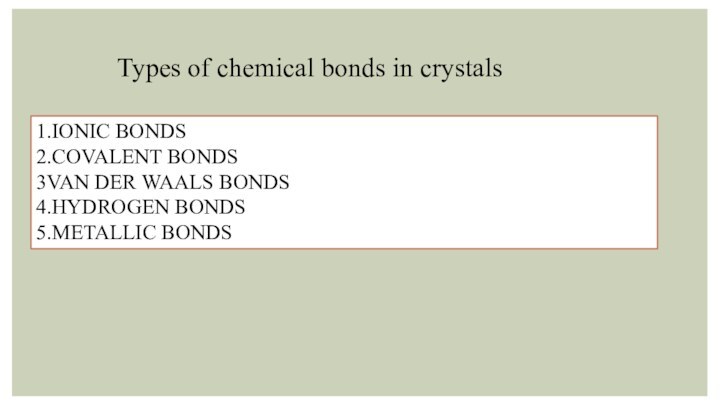
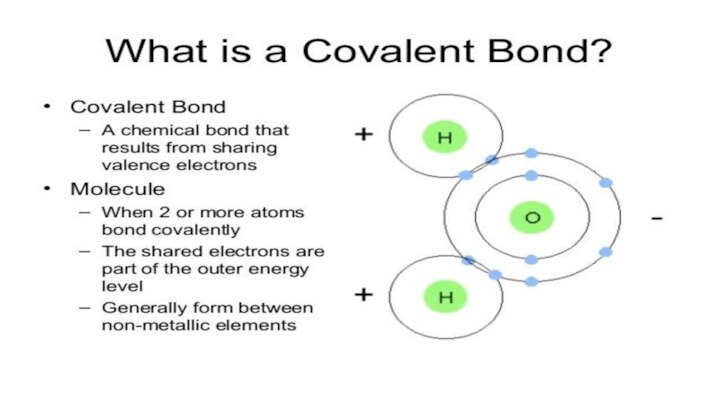
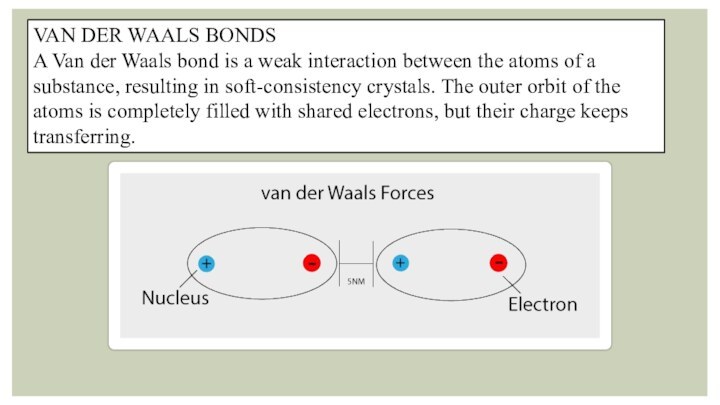
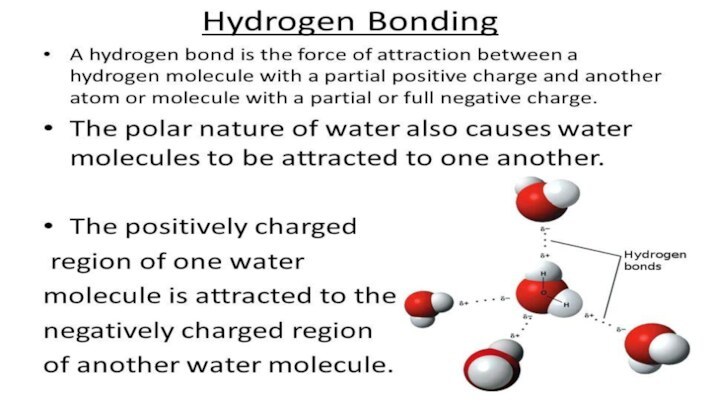
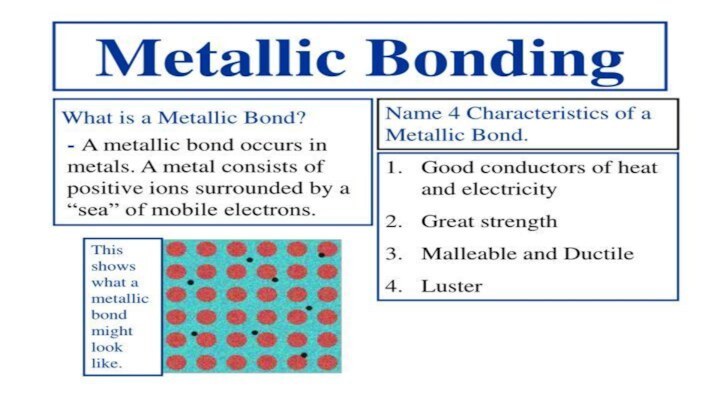
- 3. Types of chemical bonds in crystals1.IONIC BONDS2.COVALENT BONDS3VAN DER WAALS BONDS4.HYDROGEN BONDS5.METALLIC BONDS
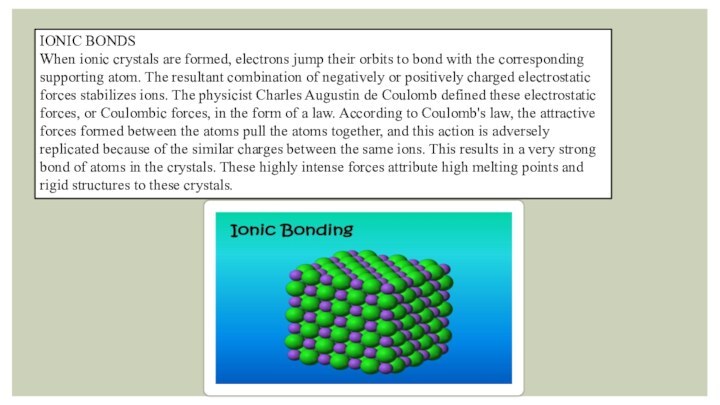
- 4. IONIC BONDSWhen ionic crystals are formed, electrons
- 6. VAN DER WAALS BONDSA Van der Waals
- 9. Скачать презентацию
- 10. Похожие презентации
Atoms bond during chemical reactions to result in crystal formation. Crystals are defined as a solid state of matter in which atoms are packed together tightly. The distinguishing feature of crystals is that their solid form