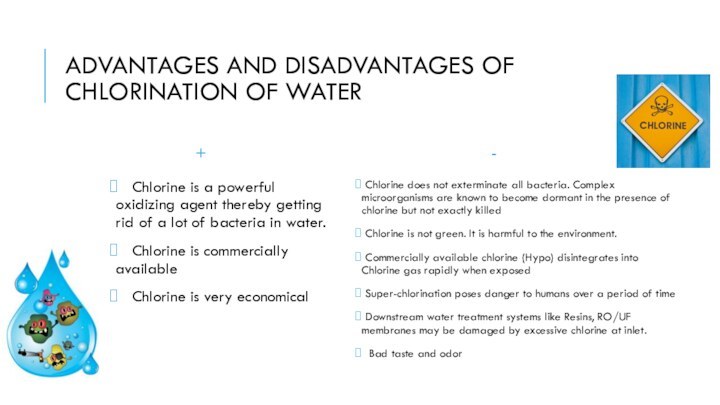
disease-causing pathogens, such as bacteria, viruses, and protozoans. The microscopic agents of
many diseases such as cholera, typhoid fever, and dysentery killed countless people annually before disinfection methods were employed routinely.Chlorine is a strong oxidizing agent
Chlorine kills via the oxidation of organic molecules. Chlorine and hydrolysis product hypochlorous acid are neutrally charged and therefore easily penetrate the negatively charged surface of pathogens. It is able to disintegrate the lipids that compose the cell wall and react with intracellular enzymes and proteins, making them nonfunctional. Microorganisms then either die or are no longer able to multiply.
When dissolved in water, chlorine converts to an equilibrium mixture of chlorine, hypochlorous acid (HOCl), and hydrochloric acid (HCl):
Cl2 + H2O ⇌ HOCl + HCl
In acidic solution, the major species are Cl2 and HOCl, whereas in alkaline solution, effectively only ClO− (hypochlorite ion) is present. Very small concentrations of ClO2−, ClO3−, ClO4− are also found.