Engineering
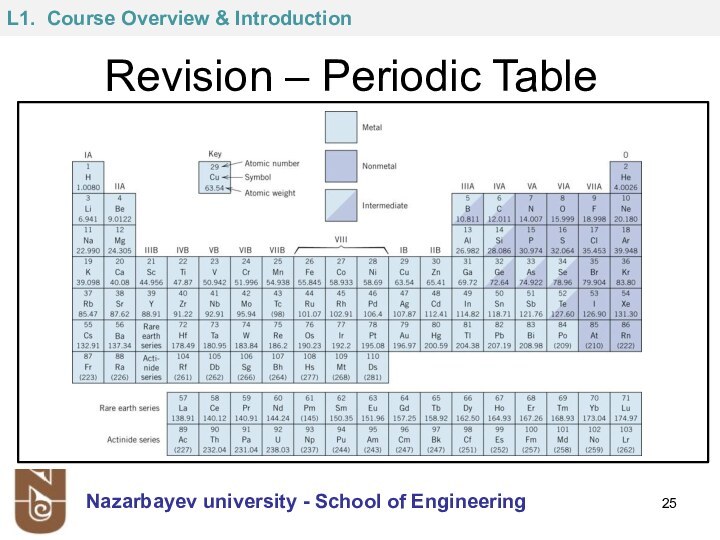
Z = Atomic number = number of protons in
nucleus
This is used to identify element
N = number of neutrons in nucleus
This is used to identify isotopes, written as (Z+N)XZ : ( e.g. 14C6 and 12C6 )
where: A = Atomic mass unit (amu)
1 amu is defined as the 1/12 of the atomic mass 12C6
Atomic mass of 12C6 is 12 amu: 6 protons (Z=6) + 6 neutrons (N=6)
This is approximately the total mass of protons + total mass of neutrons
Therefore 1 amu = Massproton ~ Massneutron = 1.67 x 10-27 kg
and A = Atomic Mass = Z + N
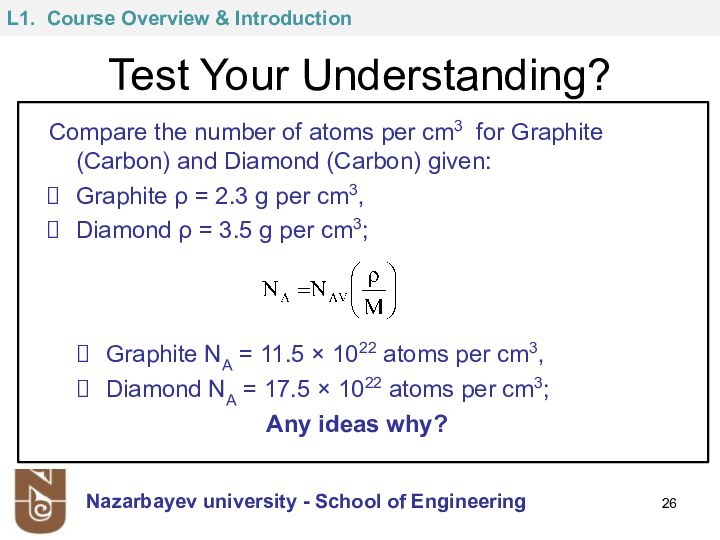
NAV = 1 mole = 6.023 x 1023 molecules or atoms (Avogadro’s number)
Atomic weight is expressed in amu/atom, i.e. 1 amu/atom = 1g/mol
L1. Course Overview & Introduction