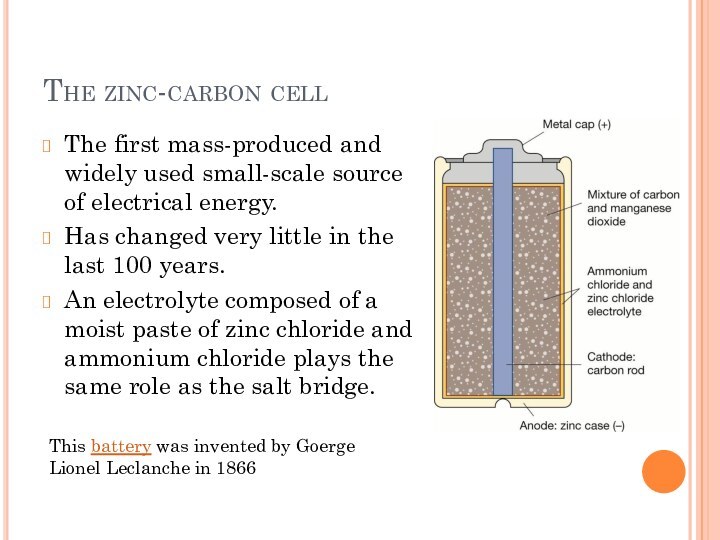
small-scale source of electrical energy.
Has changed very little in
the last 100 years.An electrolyte composed of a moist paste of zinc chloride and ammonium chloride plays the same role as the salt bridge.
This battery was invented by Goerge Lionel Leclanche in 1866