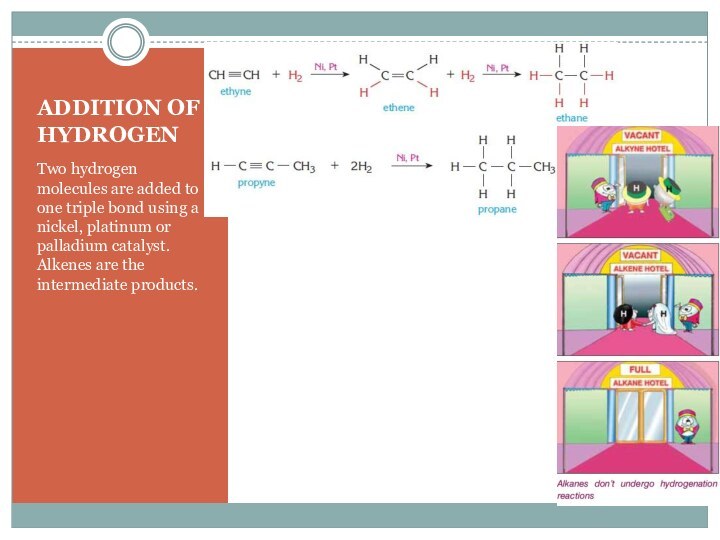
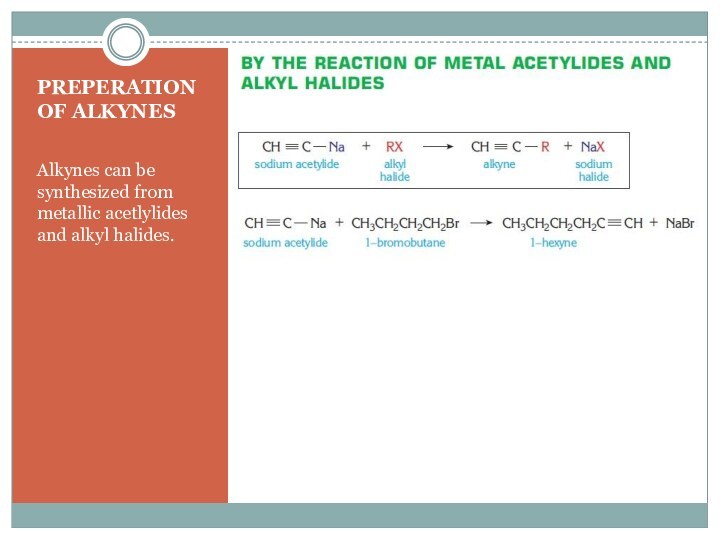
–C (triple)C- are called alkynes.
Each triple bond contains one sigma (σ) and two pi (π) bonds.
Because of the π bonds in their structure alkynes are unsaturated hydrocarbons.
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Email: Нажмите что бы посмотреть

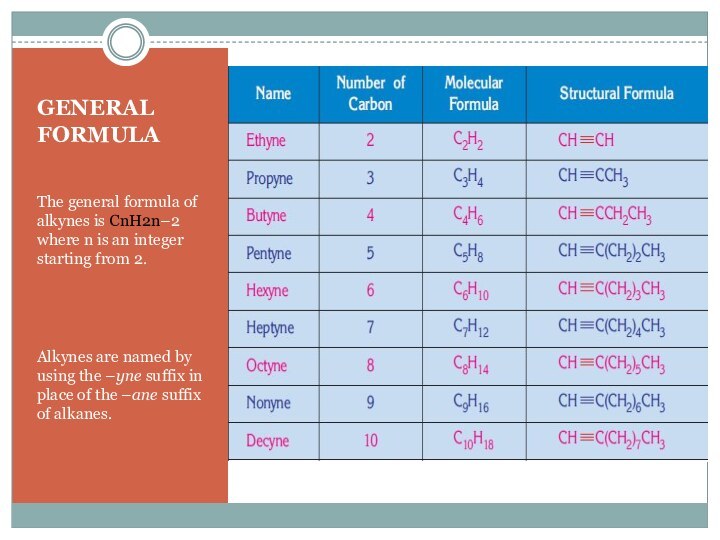
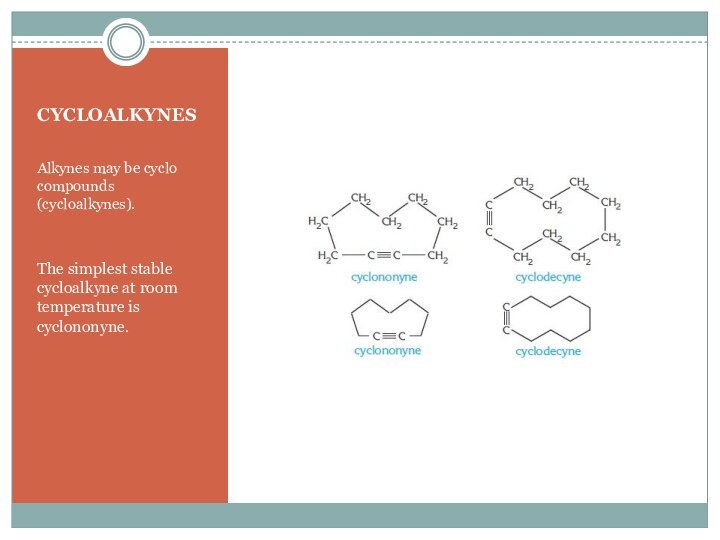
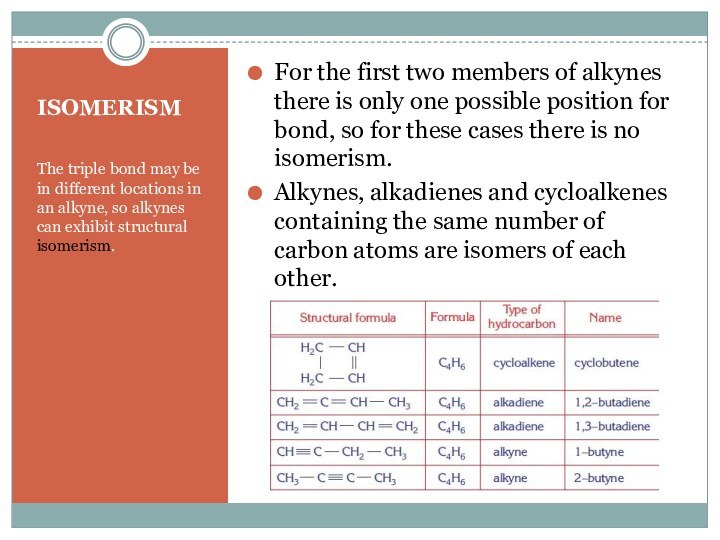





For the first two members of alkynes there is only one possible position for bond, so for these cases there is no isomerism.
Alkynes, alkadienes and cycloalkenes containing the same number of carbon atoms are isomers of each other.