different chemical reactions varies hugely. Some reactions are very
fast and others are very slow.What is the rate of these reactions?
The speed of a reaction is called the rate of the reaction.
rusting
baking
explosion
slow
fast
very fast
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Email: Нажмите что бы посмотреть


























What is the rate of these reactions?
The speed of a reaction is called the rate of the reaction.
rusting
baking
explosion
slow
fast
very fast
The rate of a reaction depends on two things:
the frequency of collisions between particles
the energy with which particles collide.
If particles collide with less energy than the activation energy, they will not react. The particles will just bounce off each other.
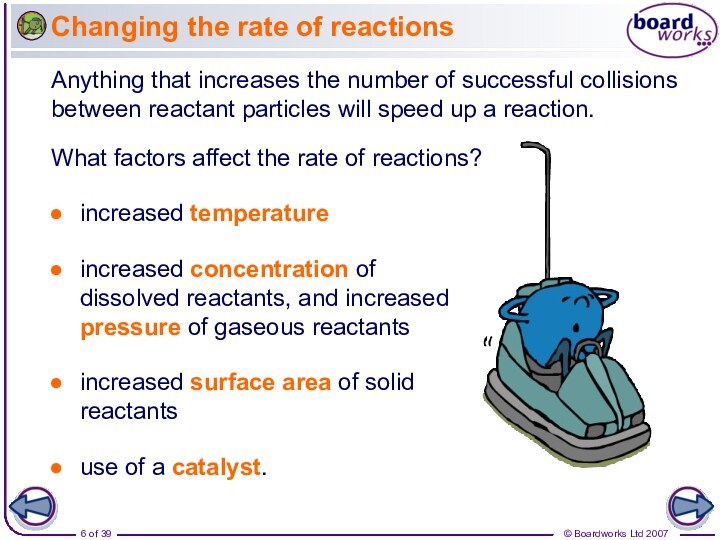
use of a catalyst.
Anything that increases the number of successful collisions between reactant particles will speed up a reaction.
What factors affect the rate of reactions?
As the reaction progresses, the concentration of reactants decreases.
This reduces the frequency of collisions between particles and so the reaction slows down.
What can be measured to calculate the rate of reaction between magnesium and hydrochloric acid?
The amount of hydrochloric acid used up (cm3/min).
The amount of magnesium chloride produced (g/min).
The amount of hydrogen product (cm3/min).
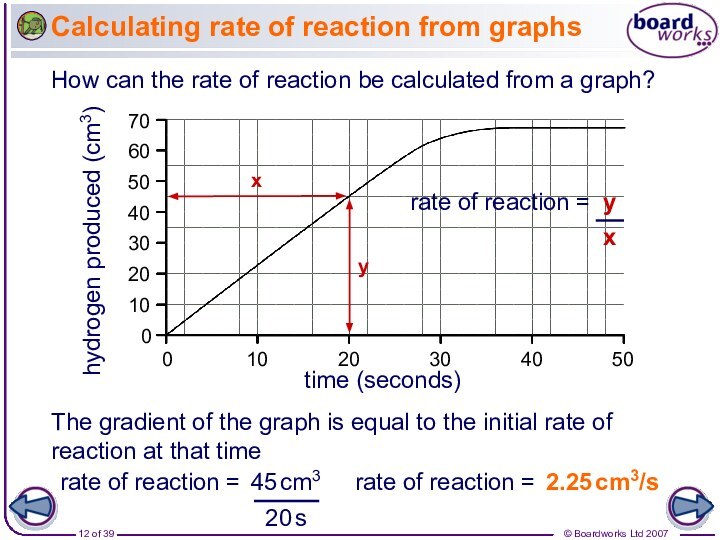
How can the rate of reaction be calculated from a graph?
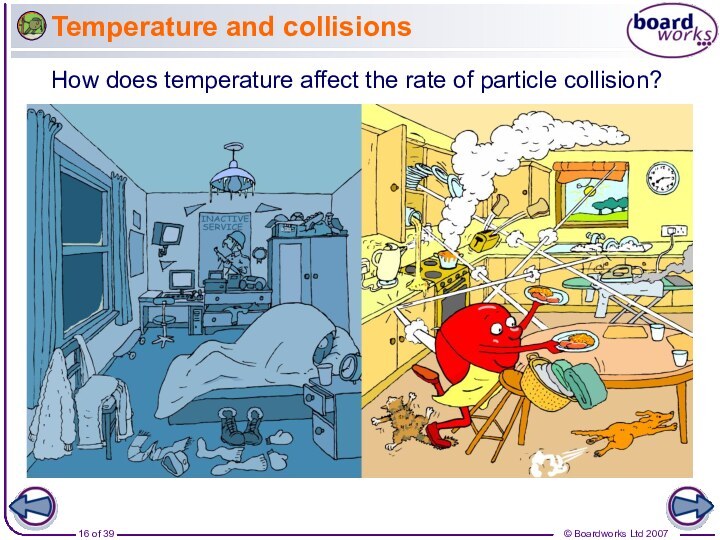
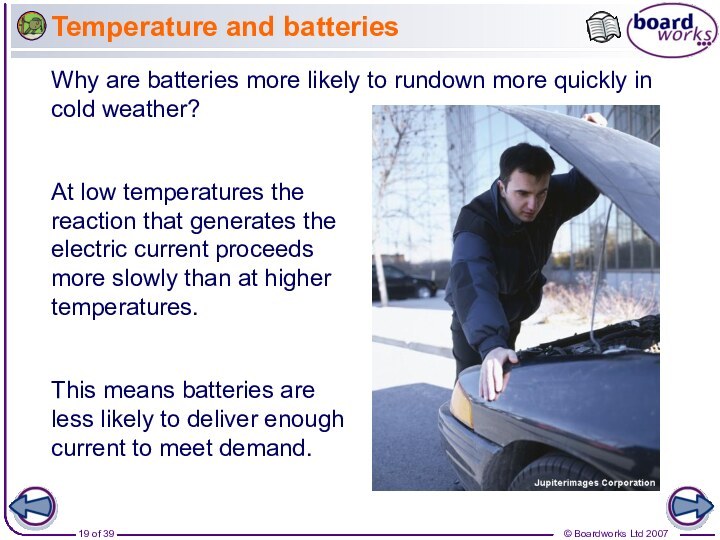
Why does increased temperature increase the rate of reaction?
At a higher temperature, particles have more energy. This means they move faster and are more likely to collide with other particles.
When the particles collide, they do so with more energy, and so the number of successful collisions increases.
This means batteries are less likely to deliver enough current to meet demand.
How can this fact be used to measure the effect of temperature on rate of reaction?
Why does increased concentration increase the rate of reaction?
At a higher concentration, there are more particles in the same amount of space. This means that the particles are more likely to collide and therefore more likely to react.
Why does increasing the pressure of gaseous reactants increase the rate of reaction?
As the pressure increases, the space in which the gas particles are moving becomes smaller.
If the solid is split into several pieces, the surface area increases. What effect will this have on rate of reaction?
The smaller the pieces, the larger the surface area. This means more collisions and a greater chance of reaction.
This means that there is an increased area for the reactant particles to collide with.
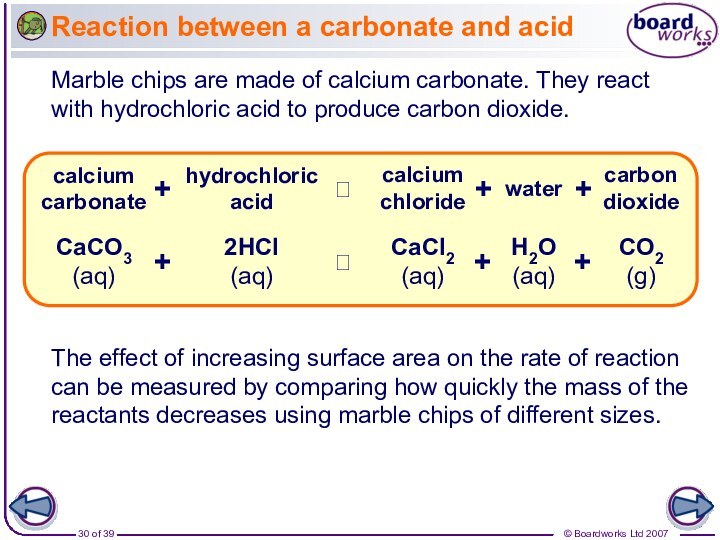
The effect of increasing surface area on the rate of reaction can be measured by comparing how quickly the mass of the reactants decreases using marble chips of different sizes.
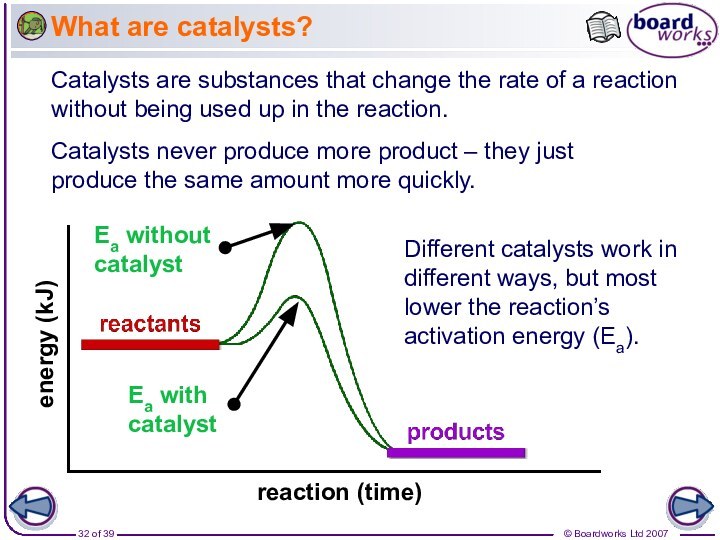
Catalysts never produce more product – they just produce the same amount more quickly.
Different catalysts work in different ways, but most lower the reaction’s activation energy (Ea).
Platinum is a catalyst in the catalytic converters of car exhausts. It catalyzes the conversion of carbon monoxide and nitrogen oxide into the less polluting carbon dioxide and nitrogen.
Iron is a catalyst in the production of ammonia from nitrogen and hydrogen (the Haber process).
Why are catalysts so important for industry?
Products can be made more quickly, saving time and money.
Catalysts reduce the need for high temperatures, saving fuel and reducing pollution.