- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Адам паппилома вирусы инфекциясына қарсы вакцинаның эффективтілігі мен қауіпсіздігі
Содержание
- 2. Тақырыбы : Адам паппилома вирусы инфекциясына қарсы вакцинаның эффективтілігі мен қауіпсіздігі
- 3. МаңыздылығыАдам паппилома вирусы кең таралған, әйелдер мен
- 4. Мақсаты: АПВ вакцинасының қаншалықты эффективтілігін, иммуногендігін және қауіпсіздігін анықтау
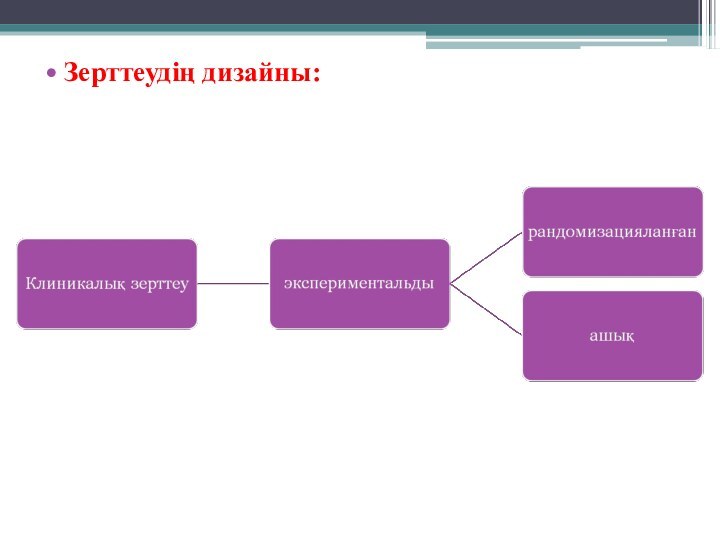
- 5. Зерттеудің дизайны:
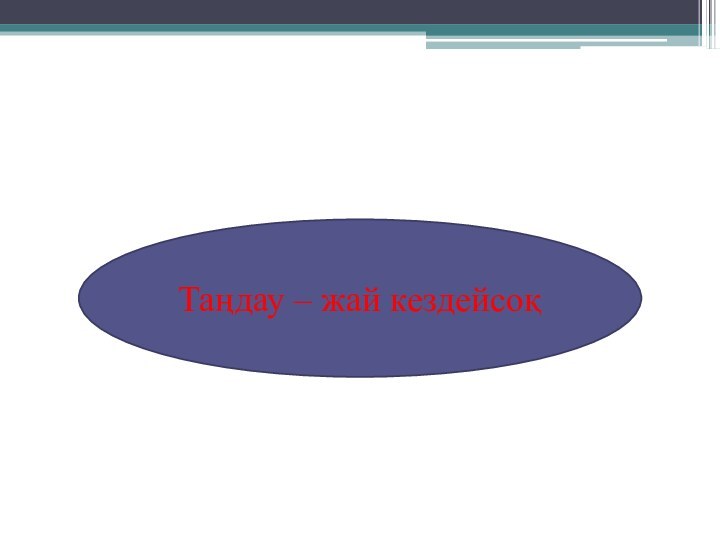
- 6. Таңдау – жай кездейсоқ
- 7. Этикалық аспектілері:Этикалық комитетпен рұқсат етілген;Зерттеу туралы толық
- 8. Сұрақ Профилактикалық мақсатта АПВ (адам паппилома вирусына)
- 9. Критерий включение:15-25 жастағы жатыр мойны патологиясыз қыз
- 10. Format: AbstractSend toJ Natl Cancer Inst. 2015 Oct 14;108(1).
- 11. Скачать презентацию
- 12. Похожие презентации
Тақырыбы : Адам паппилома вирусы инфекциясына қарсы вакцинаның эффективтілігі мен қауіпсіздігі









Слайд 2
Тақырыбы :
Адам паппилома вирусы инфекциясына қарсы вакцинаның
эффективтілігі мен қауіпсіздігі
Слайд 3
Маңыздылығы
Адам паппилома вирусы кең таралған, әйелдер мен ерлер
арасында түрлі ауру шақыратын вирус.
Қазіргі таңда 100 астам
вирустың түрлері белгілі, 80 түрі ғана зерттелген, соның ішінде 30 түрі әйел жыныс ағзаларының ауруын шақырады. Осылардың ішінде ең қауіптісі С типті АПВ. Бұл әйел жыныс мүшелерінің соның ішінде жатыр мойнының рак ауруларын шақыратын онкологиялық қауіпті вирус.
Слайд 4
Мақсаты: АПВ вакцинасының қаншалықты эффективтілігін, иммуногендігін және қауіпсіздігін
анықтау
Слайд 7
Этикалық аспектілері:
Этикалық комитетпен рұқсат етілген;
Зерттеу туралы толық ақпараттандырылған;
Ақпараттандырылған
келісім алынған;
Қатысушылар зерттеудің кез келген кезеңінде шығып кетуіне толық
құқылы;Науқасқа зиян келтірмеу;
Науқасқа пен қоғамға тиімділігі;
Слайд 8
Сұрақ
Профилактикалық мақсатта АПВ (адам паппилома вирусына) қарсы
вакцина жасау жасамауға қарағанда жатыр мойны рагының алдын алады
ма?Р- 15-25 жастағы жатыр мойны патологиясыз қыз балалар
І- АПВ вакцинасы
С- плацебо
О- Жатыр мойны рагының алдын алу
Т- 25-55 ай
Слайд 9
Критерий включение:
15-25 жастағы жатыр мойны патологиясыз қыз балалар;
Бұрын
вакцина алмаған;
Критерий исключение:
Жатыр мойны эрозиясымен бұрын ауырған;
ДЭК
ем алған;25 жастан асқан адамдар;
Слайд 10
Format: Abstract
Send to
J Natl Cancer Inst. 2015 Oct 14;108(1). pii:
djv302. doi: 10.1093/jnci/djv302. Print 2016 Jan.
Multisite HPV16/18 Vaccine Efficacy
Against Cervical, Anal, and Oral HPV Infection.Beachler DC1, Kreimer AR2, Schiffman M2, Herrero R2, Wacholder S2, Rodriguez AC2, Lowy DR2, Porras C2, Schiller JT2, Quint W2, Jimenez S2, Safaeian M2, Struijk L2, Schussler J2, Hildesheim A2, Gonzalez P2; Costa Rica HPV Vaccine Trial (CVT) Group.
Collaborators (22)
Author information
Abstract
BACKGROUND:
Previous Costa Rica Vaccine Trial (CVT) reports separately demonstrated vaccine efficacy against HPV16 and HPV18 (HPV16/18) infections at the cervical, anal, and oral regions; however, the combined overall multisite efficacy (protection at all three sites) and vaccine efficacy among women infected with HPV16 or HPV18 prior to vaccination are less known.
METHODS:
Women age 18 to 25 years from the CVT were randomly assigned to the HPV16/18 vaccine (Cervarix) or a hepatitis A vaccine. Cervical, oral, and anal specimens were collected at the four-year follow-up visit from 4186 women. Multisite and single-site vaccine efficacies (VEs) and 95% confidence intervals (CIs) were computed for one-time detection of point prevalent HPV16/18 in the cervical, anal, and oral regions four years after vaccination. All statistical tests were two-sided.
RESULTS:
The multisite woman-level vaccine efficacy was highest among "naïve" women (HPV16/18 seronegative and cervical HPV high-risk DNA negative at vaccination) (vaccine efficacy = 83.5%, 95% CI = 72.1% to 90.8%). Multisite woman-level vaccine efficacy was also demonstrated among women with evidence of a pre-enrollment HPV16 or HPV18 infection (seropositive for HPV16 and/or HPV18 but cervical HPV16/18 DNA negative at vaccination) (vaccine efficacy = 57.8%, 95% CI = 34.4% to 73.4%), but not in those with cervical HPV16 and/or HPV18 DNA at vaccination (anal/oral HPV16/18 VE = 25.3%, 95% CI = -40.4% to 61.1%). Concordant HPV16/18 infections at two or three sites were also less common in HPV16/18-infected women in the HPV vaccine vs control arm (7.4% vs 30.4%, P < .001).
CONCLUSIONS:
This study found high multisite vaccine efficacy among "naïve" women and also suggests the vaccine may provide protection against HPV16/18 infections at one or more anatomic sites among some women infected with these types prior to HPV16/18 vaccination.
TRIAL REGISTRATION:
ClinicalTrials.gov NCT00128661.
Published by Oxford University Press 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Comment in
HPV Vaccine Confers Multisite Protection. [Cancer Discov. 2015]
PMID: 26467666 PMCID: PMC4862406 DOI: 10.1093/jnci/djv302
[PubMed - indexed for MEDLINE] Free PMC Article