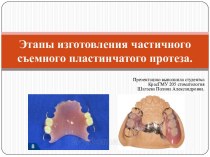
Слайд 2
What is apoptosis?
Apoptosis is a regulated cellular suicide
mechanism characterized by nuclear condensation, cell shrinkage, membrane blebbing,
and DNA fragmentation.
Apoptosis, or programmed cell death, is an evolutionary conserved genetic process of cellular suicide, which plays a crucial role in sculpting the developing organism and in “pruning” billions of unwanted, unneeded, or damaged cells every day during adult life
Слайд 3
Importance of Apoptosis
1) Crucial for embryonic development
-Errors in
Apoptosis can lead to Birth Defects
2) Important for maintaining
homeostasis
- Cell death is balanced with mitosis to regulate cell number.
3) Improper regulation contributes to human disease
- Neurodegenerative diseases
Parkinson’s
Alzheimer’s
-Cancer
- Autoimmune diseases e.g. (diabetes type I)
- Viral diseases
Слайд 4
Morphology
Cell shrinkage (condensation of cytoplasm)
Breakdown of mitochondria; release
of cytochrome C
Nuclear condensation
Nuclear fragmentation
Cell membrane blebbing
Fragmentation; apoptotic body
formation: membrane-bound cellular fragments, which often lack nuclei
Phagocytosis
Слайд 5
How Apoptosis Differs from Necrosis?
Apoptosis is intrinsically controlled,
necrosis is not
Apoptosis is more rapid (12-24 hours) than
necrosis
Apoptosis is induced by endogenous or exogenous stimuli, necrosis is always induced by exogenous harms
Apoptosis is limited to single or few cells at a time, and occurs among healthy cell population, necrosis is usually more extensive & occurs in tissue exposed to injuries
Cell cytoplasm shrinks in apoptosis and swells in necrosis.
Nucleosomes of apoptotic cells are 180 bp fragments, contrary to the irregular ones in necrosis
Apoptosis has no inflammation, while necrosis leads to liberation of pro-inflammatory mediators
Apoptosis has no systemic manifestations contrary to most inflammations
Слайд 6
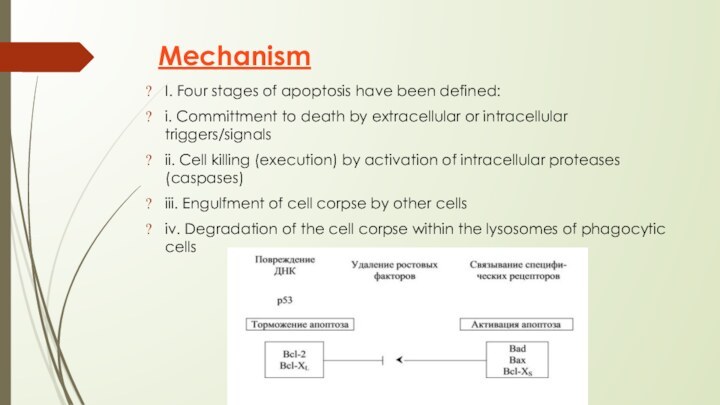
Mechanism
I. Four stages of apoptosis have been defined:
i. Committment to death by extracellular or intracellular triggers/signals
ii.
Cell killing (execution) by activation of intracellular proteases (caspases)
iii. Engulfment of cell corpse by other cells
iv. Degradation of the cell corpse within the lysosomes of phagocytic cells
Слайд 7
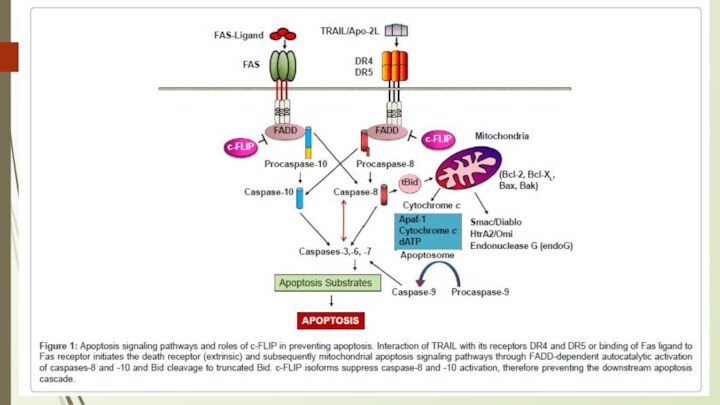
Death Factors
Definition: cytokines that activate an apoptosis program
by binding to their specific receptor.
Typical examples of
death factors are:
Fasligand, FAS L
TNF (tumor necrosis factor) and
TRAIL (TNF-related apoptosis-inducing ligand).
- Apoptosis can also be induced by cytotoxic T-lymphocytes using the enzyme granzyme.
Слайд 8
III. Activation of Caspase cascade
i. Various stimuli
described above eventually activate the executioner (caspase) cascade
ii.
At least 14 different caspases exist in human cells
iii. Caspase cascades are apparently required for complete execution
Слайд 9
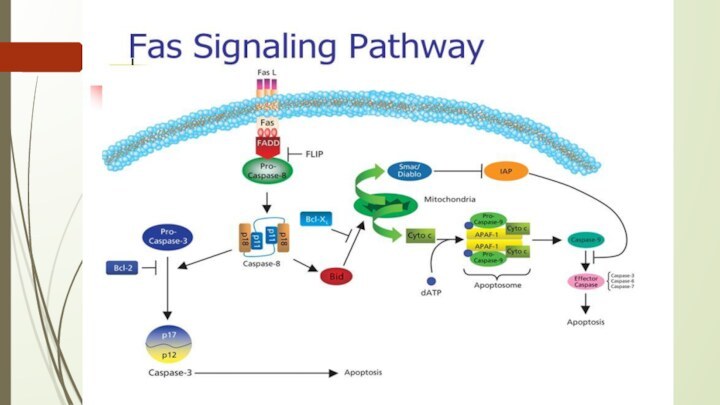
The intrinsic (mitochondrial) pathway of apoptosis.
Death agonists cause
changes in the inner mitochondrial membrane, resulting in the
mitochondrial permeability transition (MPT) and release of cytochrome c and other pro-apoptotic proteins into the cytosol, which activate caspases.
AIF= Apoptosis inhibitory factor;
IAPs= Inhibitors of apoptosis proteins;
Apaf-1= apoptosis protease activating factor
Слайд 10
Caspases are central initiators and executioners of apoptosis
The
term caspases is derived from cysteine-dependent aspartate-specific proteases
The caspase
cascade can be activated by:
Granzyme B released by cytotoxic T lymphocytes which is known to activate caspase-3 and -7;
death receptors (like FAS, TRAIL receptors and TNF receptor) which can activate caspase-8 and -10; and
the apoptosome, regulated by cytochrome c and the Bcl-2 family, which activates caspase-9.
Слайд 17
Resistance to Fas signalling in cancer
Слайд 18
Ингибиторы апоптоза (антиапоптические факторы). К наиболее серьезным ингибиторам
апоптоза относятся ростовые факторы. Другие: нейтральные аминокислоты, цинк, эстрогены,
андрогены, некоторые белки.
Пример: Белки семейства 1АР — подавляют активность каспаз 3 и 9, один из этих белков (Survin) обнаружен в опухолевых клетках. С ним связывают резистентность опухолевых клеток к химиотерапии.
Активаторы апоптоза(проапоптические факторы). Это проапоптические гены и их продукция: гены семейства BCL-2 (ВАХ и BID); гены Rb и Р53 (запускают апоптоз, если клетка задержана механизмом checkpoint).
Патогенез многих заболеваний, в том числе и опухолевых, связан со снижением способности клеток подвергаться апоптозу. Отсюда накопление поврежденных клеток и формирование опухоли.
Слайд 19
Bcl-2
Bcl2 was the first apoptosis-related gene that was
recognized to play a role in tumorigenesis, and indeed,
Bcl-2 is overexpressed in a variety of cancers, contributing to cancer cell survival through direct inhibition of apoptosis.
BCL-2 is a human proto-oncogene located on chromosome 18.
Its product is an integral membrane protein (called Bcl-2) located in the membranes of the endoplasmic reticulum (ER), nuclear envelope, and in the outer membrane of the mitochondria.
The gene was discovered as the translocated locus in a B-cell leukemia (hence the name). This translocation is also found in some B-cell lymphomas.
Слайд 20
Белки супрессоры
1. Обнаружение повреждения в структуре ДНК.
Этот факт - стимул для активации генов-супрессоров.
2. Гены-супрессоры продуцируют
белки Rb и р53.
3.Белки Rb и р53 запускают апоптоз поврежденной клетки. Это - индукторы апоптоза. Белок р53 индуцирует апоптоз в момент G1/S. Белок Rb индуцирует апоптоз в момент G2/M.
Слайд 21
Биологическая роль генов-супрессоров: они не пропускают в митоз
клетку с поврежденной ДНК. Дефект гена-супрессора ведет к размножению
поврежденной клетки. Пролиферация поврежденной клетки - основа опухолевого роста.
Наследование генов-супрессоров. В каждой клетке есть по два аллеля любых генов. Значит, в каждой клетке есть два гена-супрессора. Дефект одного гена-супрессора повышает риск пропуска в митоз поврежденной клетки. Дефект обоих генов-супрессоров всегда приводит к пропуску в митоз поврежденной клетки и опухолевому росту.
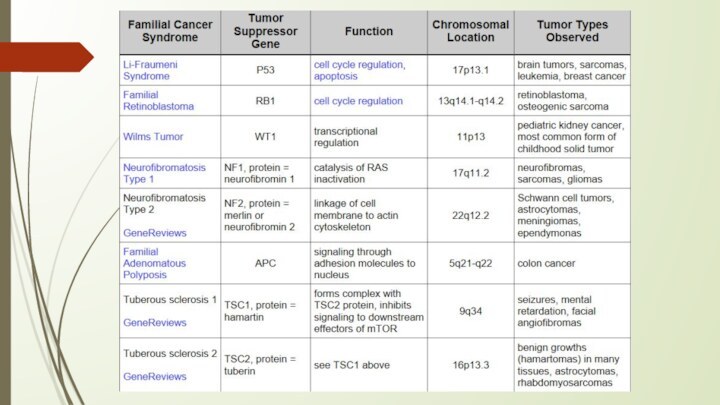
Пример: наследственная ретинобластома - опухоль сетчатки глаза - диагностируется в раннем детском возрасте (зрачок отсвечивает красным). Этиология - наследственный дефект гена-супрессора Rb и как следствие - постоянный пропуск в митоз клеток с поврежденной ДНК.
Слайд 23
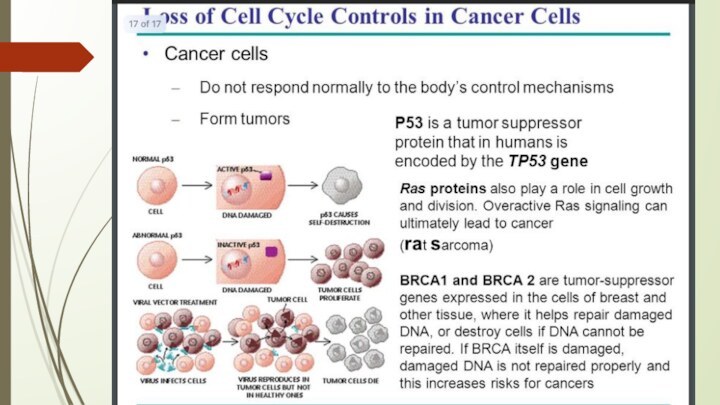
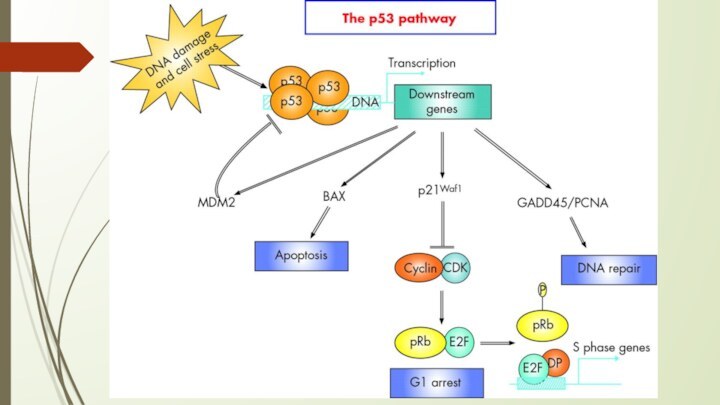
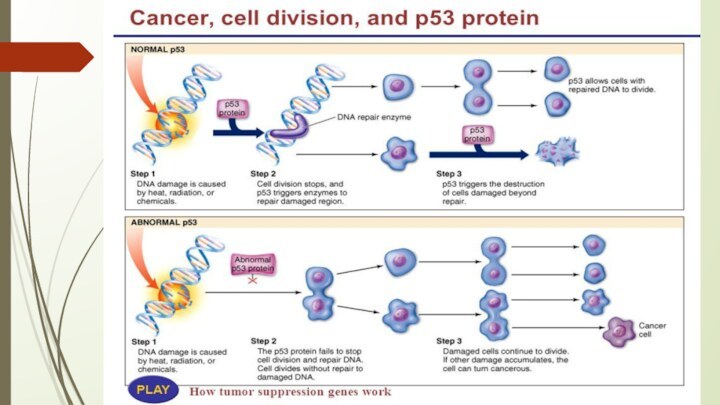
P53 protein
Acts as a tumor suppressor gene
2 Main
Functions:
halts growth and division in cell cycle under
aberrant conditions
induces apoptosis
Loss of p53 function leading cause in 30-50% of various types of cancers
Слайд 24
Сигнальный путь № 1 (связан с повреждением ДНК):
1.
Повреждение ДНК
2. Активация гена р53 и продукция соответствующего белка
3.
Активация проапоптических генов семейства BCL-2 (ВАХ и BID)
4. Образование белков этих генов
5. Активация каспазы 9
6. Активация каспазы 3
7. Активация других каспаз и протеаз
8. Апоптоз
Слайд 25
The p53 gene like the Rb gene, is

a tumor suppressor gene, i.e., its activity stops the
formation of tumors. If a person inherits only one functional copy of the p53 gene from their parents, they are predisposed to cancer and usually develop several independent tumors in a variety of tissues in early adulthood. This condition is rare, and is known as Li-Fraumeni syndrome. However, mutations in p53 are found in most tumor types, and so contribute to the complex network of molecular events leading to tumor formation.
The p53 gene has been mapped to chromosome 17. In the cell, p53 protein binds DNA, which in turn stimulates another gene to produce a protein called p21 that interacts with a cell division-stimulating protein (cdk2). When p21 is complexed with cdk2 the cell cannot pass through to the next stage of cell division. Mutant p53 can no longer bind DNA in an effective way, and as a consequence the p21 protein is not made available to act as the 'stop signal' for cell division. Thus cells divide uncontrollably, and form tumors.
Слайд 35
https://www.youtube.com/watch?v=8kbAQq_Pp8gb - Intrinsic Pathway
https://www.youtube.com/watch?v=Aqf-n3pHv1I – Induction of apoptosis
https://www.youtube.com/watch?v=1_s7KS2rit4
– Role of Mitochondria on apoptosis
https://www.youtube.com/watch?v=Rlk9ZzInzuA – Extrinsic Pathway/
TNF
https://www.youtube.com/watch?v=f8CpWl-Tqf8 – E/Fas ligand
Слайд 36
Literature:
https://www.researchgate.net/publication/221742318_Targeting_the_Fas-FasL_signaling_pathway_in_cancer_therapy
https://www.cambridge.org/core/books/molecular-oncology/induction-of-apoptosis/0E2E934B7A64CCF86992F04BF081D8C2#fndtn-information
https://www.cellsignal.com/contents/science-cst-pathways-apoptosis/regulation-of-apoptosis-interactive-pathway/pathways-apoptosis-regulation
https://www.nature.com/articles/7290060
https://www.ncbi.nlm.nih.gov/books/NBK22268/ p53
https://themedicalbiochemistrypage.org/tumor-suppressors.php
Слайд 37
http://humbio.ru/humbio/cytology/00118f2b.htm p53