- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему In vitro Diagnosis of Drug Allergy: Current Status and Perspectives
Содержание
- 2. Dr. Fleisher has no conflicts of interest related to this presentation
- 3. Drugs as ImmunogensBiologics: foreign macromolecules (e.g. antibodies,
- 4. Use of in vitro Testing for Drug
- 5. Immediate Reaction to DrugGell and Coombs type
- 6. Tryptase TestingMature tryptase reflects mast cell degranulation
- 7. Allergen Specific IgE TestingIn vitro “equivalent” of
- 8. Basophil Activation TestTest evaluates basophils present in
- 9. Basophil Activation TestSteiner, M. et al. J
- 10. Basophil Activation TestAdvantagesDoes not subject patient to
- 11. BAT in Radiocontrast Media ReactionsEvaluation of 26
- 12. Delayed Immunologic Reaction to DrugsMost commonly linked
- 13. Focus of in vitro Testing Confirm that
- 14. Varied concentrations of pure drug, incubate at
- 15. Lymphocyte Transformation Test (LTT)Must use controls to
- 16. Evaluation of LTT in Different Types of
- 17. LTT Used to Identify the Drug
- 18. LTT SummaryLTT appears to be a suitable
- 19. Alternatives to LTT (3H Thymidine)Evaluation of upregulation
- 20. Varied concentrations of pure drug, incubate at
- 21. CD69 Upregulation in Response to DrugEvaluation of
- 22. Summary of LTT AlternativesCD69 upregulation appears to
- 23. Immunopathogenesis of SJS/TEN Bullous skin processes (SJS/TEN) associated
- 24. “Real Time” Test to Diagnose SJS/TENThe serum
- 25. In the FutureMultiplex cytokine evaluation following in
- 26. Summary in vitro Testing in Drug Allergy
- 27. ConclusionsThe clinical story remains the most important
- 28. Скачать презентацию
- 29. Похожие презентации








Слайд 3
Drugs as Immunogens
Biologics: foreign macromolecules (e.g. antibodies, recombinant
proteins) act directly as immunogen
Drugs (non-biologics)
Hapten – drug (e.g.
β-lactam antibiotics, quinidine) combines with a host macromoleculePro-hapten – processed drug (e.g. sulfonamides, phenytoin) combines with a host macromolecule
Drugs can act directly to stimulate an immune receptor (pharmacologic interaction with immune receptors = p-i concept)
Слайд 4
Use of in vitro Testing for Drug Allergy
Testing
in the setting of an immediate drug reaction
Testing in
the setting of a delayed drug reactionTesting on the horizon
Слайд 5
Immediate Reaction to Drug
Gell and Coombs type 1
reaction that occurs rapidly upon exposure to a specific
drugStandard approach to evaluate is immediate skin testing (penicillin major and minor determinants are validated, other drugs ?)
In vitro methods of evaluation include:
Tryptase to establish mast cell degranulation
Allergen (drug) specific IgE testing
Basophil activation test (BAT)
Слайд 6
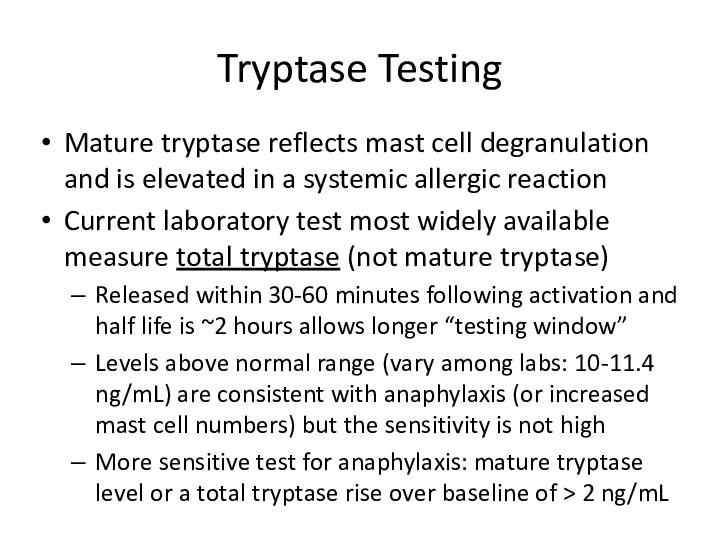
Tryptase Testing
Mature tryptase reflects mast cell degranulation and
is elevated in a systemic allergic reaction
Current laboratory test
most widely available measure total tryptase (not mature tryptase)Released within 30-60 minutes following activation and half life is ~2 hours allows longer “testing window”
Levels above normal range (vary among labs: 10-11.4 ng/mL) are consistent with anaphylaxis (or increased mast cell numbers) but the sensitivity is not high
More sensitive test for anaphylaxis: mature tryptase level or a total tryptase rise over baseline of > 2 ng/mL
Слайд 7
Allergen Specific IgE Testing
In vitro “equivalent” of immediate
skin testing
Does not subject patient to risk and does
not have a potential of inducing sensitization Limited range of drugs available impacts utility: β-lactams (penicilloyl G & V, ampicilloyl, amoxocilloyl), ACTH, cefator, ceftriazone, chlorhexidene, ethylene oxide, gelatin, insulin, neuromuscular blocking agents, tetanus toxoid)
Tests generally have high specificity with lower sensitivity - negative test does not rule out allergy
Слайд 8
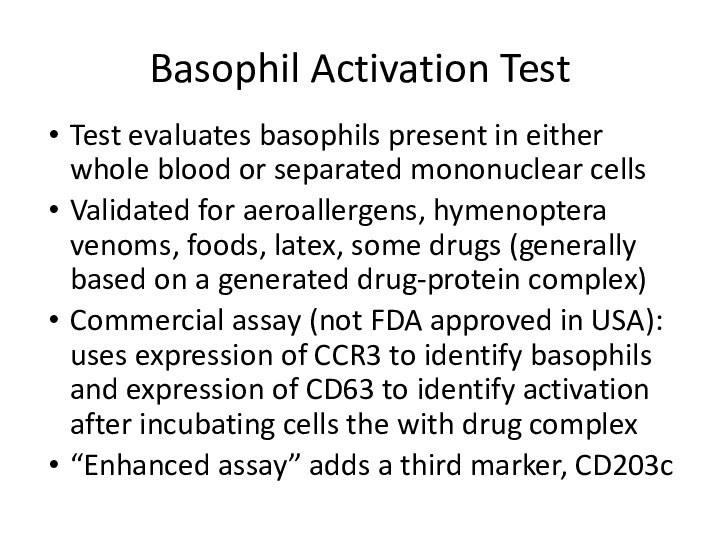
Basophil Activation Test
Test evaluates basophils present in either
whole blood or separated mononuclear cells
Validated for aeroallergens, hymenoptera
venoms, foods, latex, some drugs (generally based on a generated drug-protein complex)Commercial assay (not FDA approved in USA): uses expression of CCR3 to identify basophils and expression of CD63 to identify activation after incubating cells the with drug complex
“Enhanced assay” adds a third marker, CD203c
Слайд 9
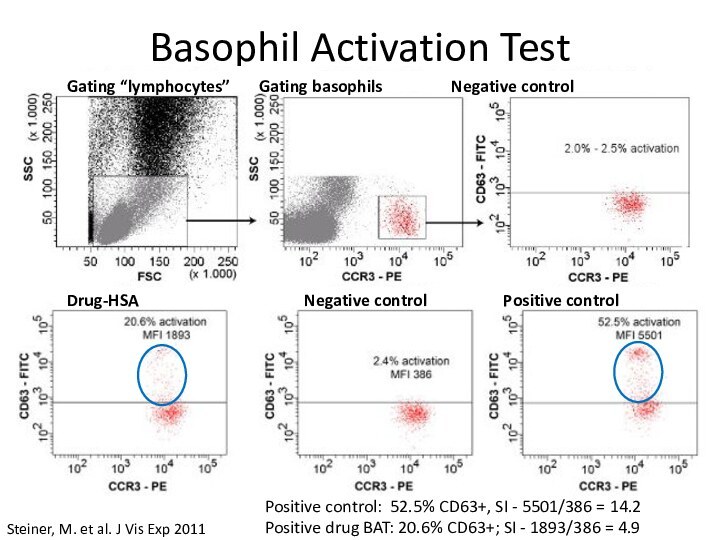
Basophil Activation Test
Steiner, M. et al. J Vis
Exp 2011
Gating “lymphocytes” Gating basophils
Negative controlDrug-HSA Negative control Positive control
Positive control: 52.5% CD63+, SI - 5501/386 = 14.2
Positive drug BAT: 20.6% CD63+; SI - 1893/386 = 4.9
Слайд 10
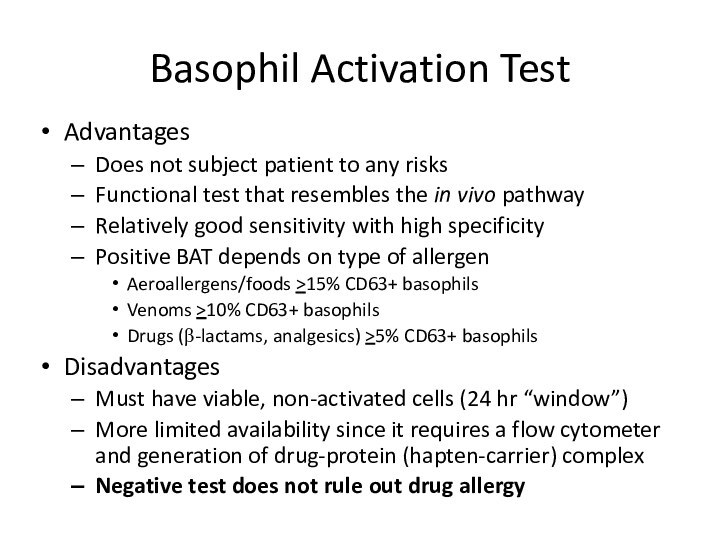
Basophil Activation Test
Advantages
Does not subject patient to any
risks
Functional test that resembles the in vivo pathway
Relatively good
sensitivity with high specificityPositive BAT depends on type of allergen
Aeroallergens/foods >15% CD63+ basophils
Venoms >10% CD63+ basophils
Drugs (β-lactams, analgesics) >5% CD63+ basophils
Disadvantages
Must have viable, non-activated cells (24 hr “window”)
More limited availability since it requires a flow cytometer and generation of drug-protein (hapten-carrier) complex
Negative test does not rule out drug allergy
Слайд 11
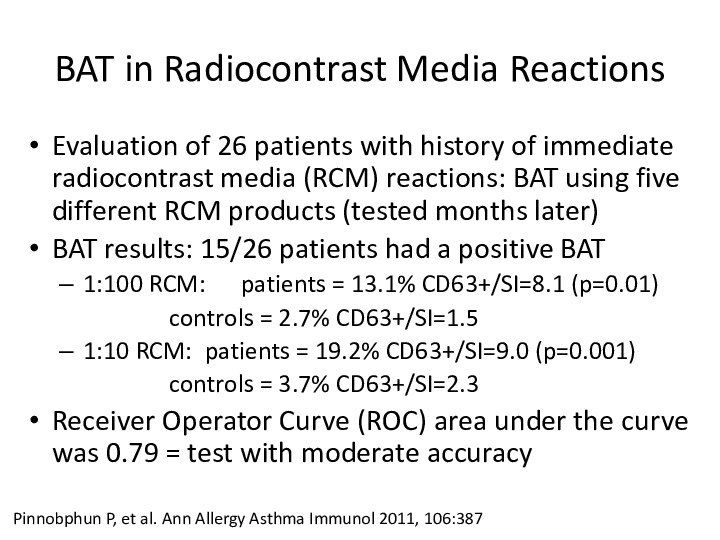
BAT in Radiocontrast Media Reactions
Evaluation of 26 patients
with history of immediate radiocontrast media (RCM) reactions: BAT
using five different RCM products (tested months later)BAT results: 15/26 patients had a positive BAT
1:100 RCM: patients = 13.1% CD63+/SI=8.1 (p=0.01)
controls = 2.7% CD63+/SI=1.5
1:10 RCM: patients = 19.2% CD63+/SI=9.0 (p=0.001)
controls = 3.7% CD63+/SI=2.3
Receiver Operator Curve (ROC) area under the curve was 0.79 = test with moderate accuracy
Pinnobphun P, et al. Ann Allergy Asthma Immunol 2011, 106:387
Слайд 12
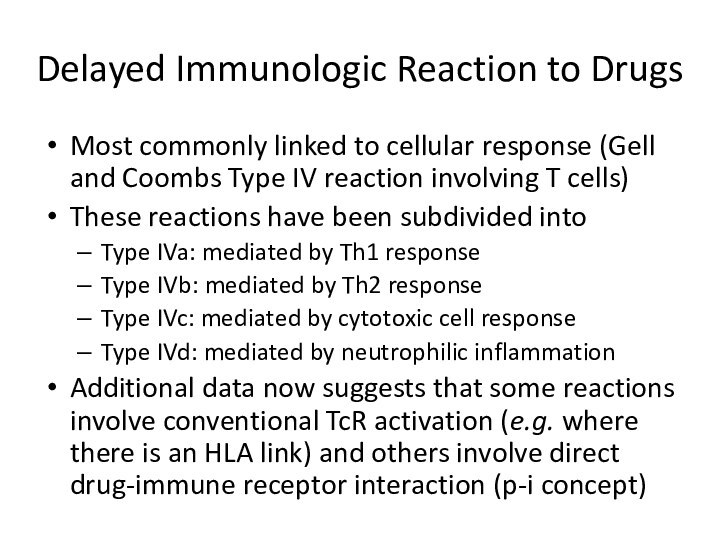
Delayed Immunologic Reaction to Drugs
Most commonly linked to
cellular response (Gell and Coombs Type IV reaction involving
T cells)These reactions have been subdivided into
Type IVa: mediated by Th1 response
Type IVb: mediated by Th2 response
Type IVc: mediated by cytotoxic cell response
Type IVd: mediated by neutrophilic inflammation
Additional data now suggests that some reactions involve conventional TcR activation (e.g. where there is an HLA link) and others involve direct drug-immune receptor interaction (p-i concept)
Слайд 13
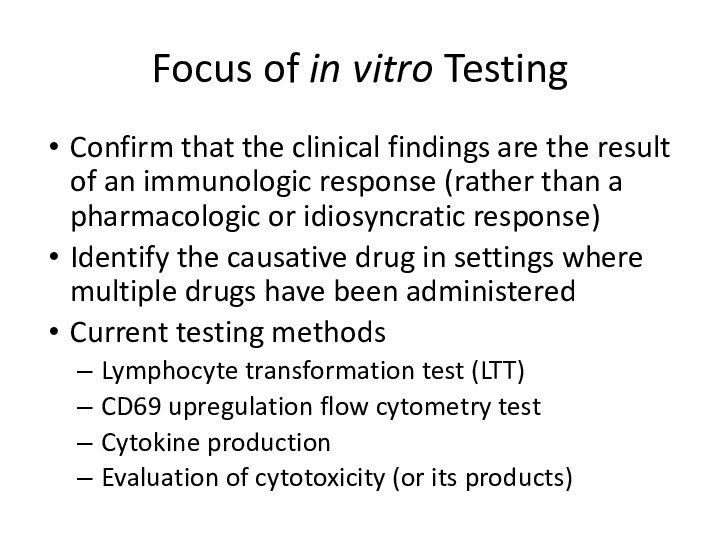
Focus of in vitro Testing
Confirm that the
clinical findings are the result of an immunologic response
(rather than a pharmacologic or idiosyncratic response)Identify the causative drug in settings where multiple drugs have been administered
Current testing methods
Lymphocyte transformation test (LTT)
CD69 upregulation flow cytometry test
Cytokine production
Evaluation of cytotoxicity (or its products)
Слайд 14
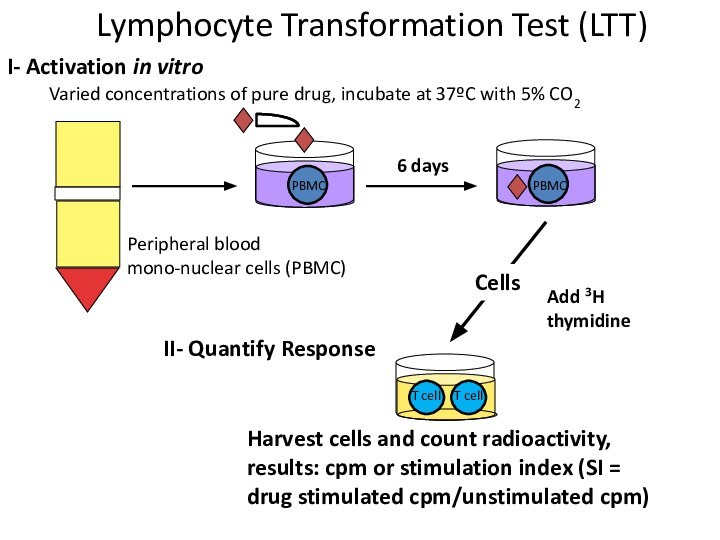
Varied concentrations of pure drug, incubate at 37ºC
with 5% CO2
Peripheral blood mono-nuclear cells (PBMC)
I- Activation in
vitroII- Quantify Response
Harvest cells and count radioactivity, results: cpm or stimulation index (SI = drug stimulated cpm/unstimulated cpm)
PBMC
PBMC
Cells
Lymphocyte Transformation Test (LTT)
Add 3H thymidine
T cell
T cell
6 days
Слайд 15
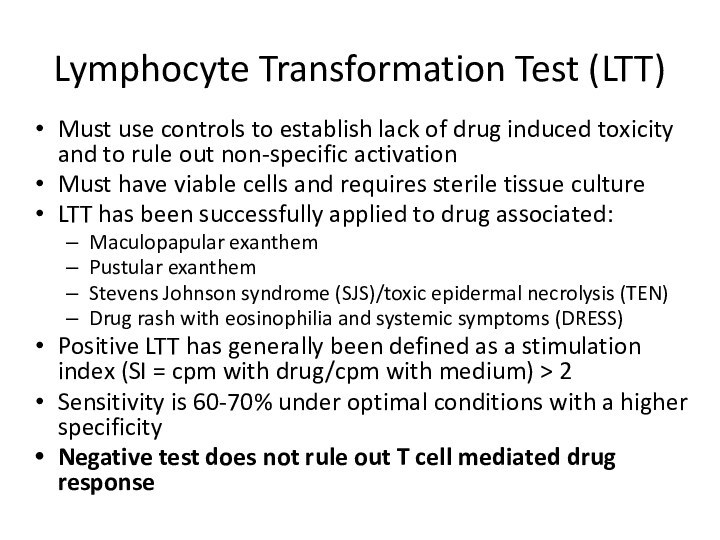
Lymphocyte Transformation Test (LTT)
Must use controls to establish
lack of drug induced toxicity and to rule out
non-specific activationMust have viable cells and requires sterile tissue culture
LTT has been successfully applied to drug associated:
Maculopapular exanthem
Pustular exanthem
Stevens Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN)
Drug rash with eosinophilia and systemic symptoms (DRESS)
Positive LTT has generally been defined as a stimulation index (SI = cpm with drug/cpm with medium) > 2
Sensitivity is 60-70% under optimal conditions with a higher specificity
Negative test does not rule out T cell mediated drug response
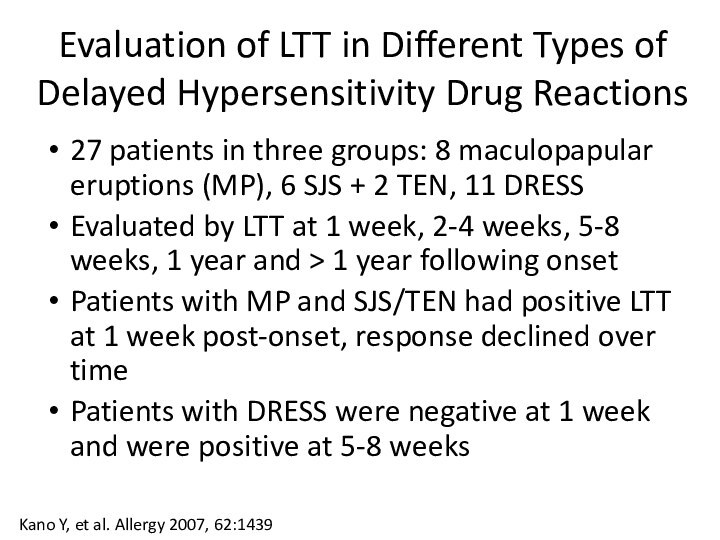
Слайд 16 Evaluation of LTT in Different Types of Delayed
Hypersensitivity Drug Reactions
27 patients in three groups: 8 maculopapular
eruptions (MP), 6 SJS + 2 TEN, 11 DRESSEvaluated by LTT at 1 week, 2-4 weeks, 5-8 weeks, 1 year and > 1 year following onset
Patients with MP and SJS/TEN had positive LTT at 1 week post-onset, response declined over time
Patients with DRESS were negative at 1 week and were positive at 5-8 weeks
Kano Y, et al. Allergy 2007, 62:1439
Слайд 17 LTT Used to Identify the Drug that Induced
DRESS
Two patients receiving multiple drugs including anticonvulsants and antibiotics
associated with the development of DRESSEvaluation by LTT utilized all drugs that had been given, each at 7 concentrations (1-200 μg/ml)
Studied 3 months after the clinical presentation
Causative drug was identified as ceftriaxone in one pt and piperacillin-tazobactam in the other pt
LTT assay proved valuable in defining the drug associated with DRESS (avoid in the future)
Jurado-Palomo J, et al. J Investig Allergol Clin Immunol 2010, 20:433
Слайд 18
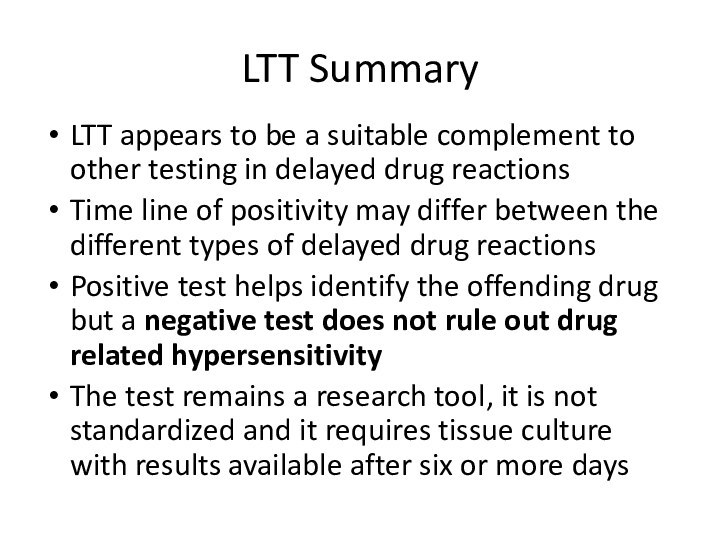
LTT Summary
LTT appears to be a suitable complement
to other testing in delayed drug reactions
Time line of
positivity may differ between the different types of delayed drug reactionsPositive test helps identify the offending drug but a negative test does not rule out drug related hypersensitivity
The test remains a research tool, it is not standardized and it requires tissue culture with results available after six or more days
Слайд 19
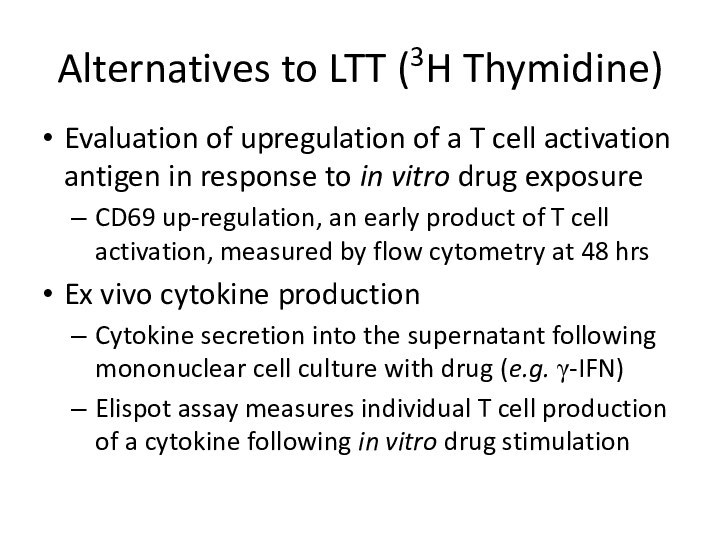
Alternatives to LTT (3H Thymidine)
Evaluation of upregulation of
a T cell activation antigen in response to in
vitro drug exposureCD69 up-regulation, an early product of T cell activation, measured by flow cytometry at 48 hrs
Ex vivo cytokine production
Cytokine secretion into the supernatant following mononuclear cell culture with drug (e.g. γ-IFN)
Elispot assay measures individual T cell production of a cytokine following in vitro drug stimulation
Слайд 20
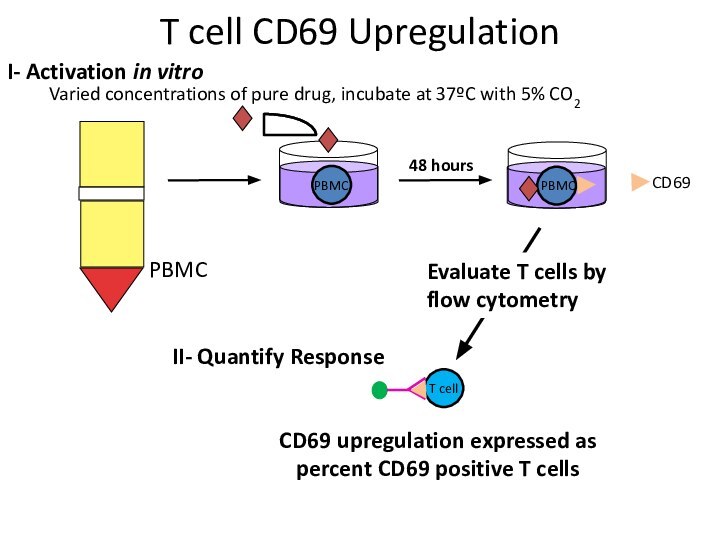
Varied concentrations of pure drug, incubate at 37ºC
with 5% CO2
PBMC
I- Activation in vitro
II- Quantify Response
PBMC
PBMC
Evaluate T
cells by flow cytometryCD69 upregulation expressed as percent CD69 positive T cells
T cell CD69 Upregulation
T cell
CD69
48 hours
Слайд 21
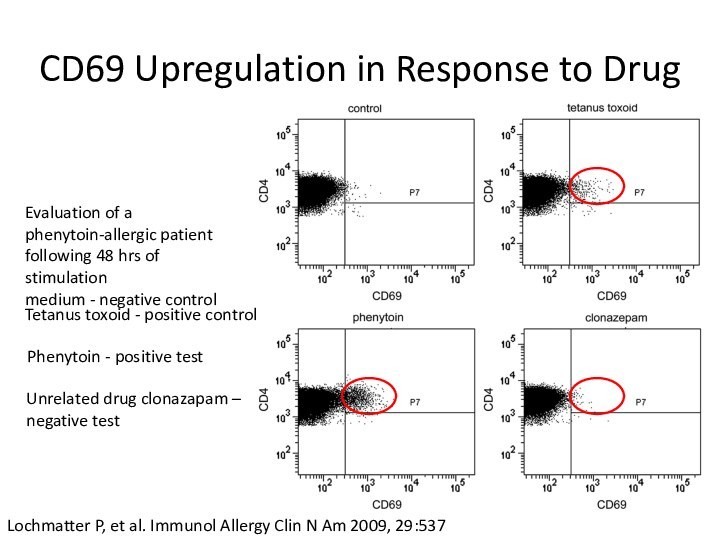
CD69 Upregulation in Response to Drug
Evaluation of a
phenytoin-allergic patient following 48 hrs of stimulation
medium -
negative controlLochmatter P, et al. Immunol Allergy Clin N Am 2009, 29:537
Tetanus toxoid - positive control
Phenytoin - positive test
Unrelated drug clonazapam –
negative test
Слайд 22
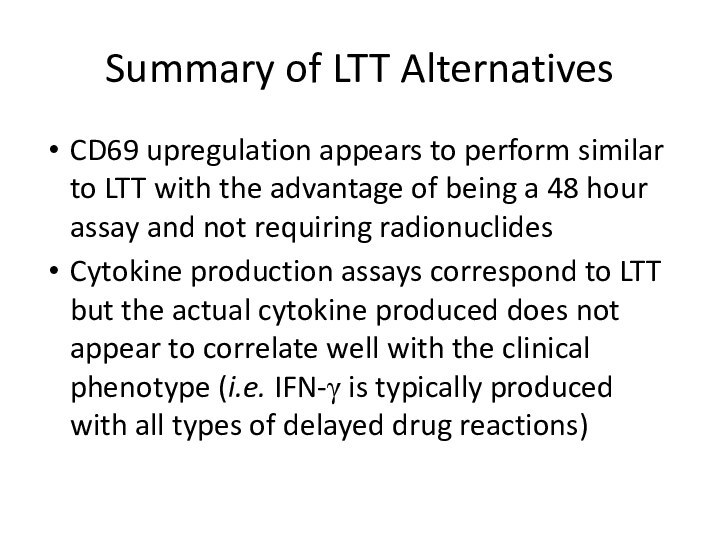
Summary of LTT Alternatives
CD69 upregulation appears to perform
similar to LTT with the advantage of being a
48 hour assay and not requiring radionuclidesCytokine production assays correspond to LTT but the actual cytokine produced does not appear to correlate well with the clinical phenotype (i.e. IFN-γ is typically produced with all types of delayed drug reactions)
Слайд 23
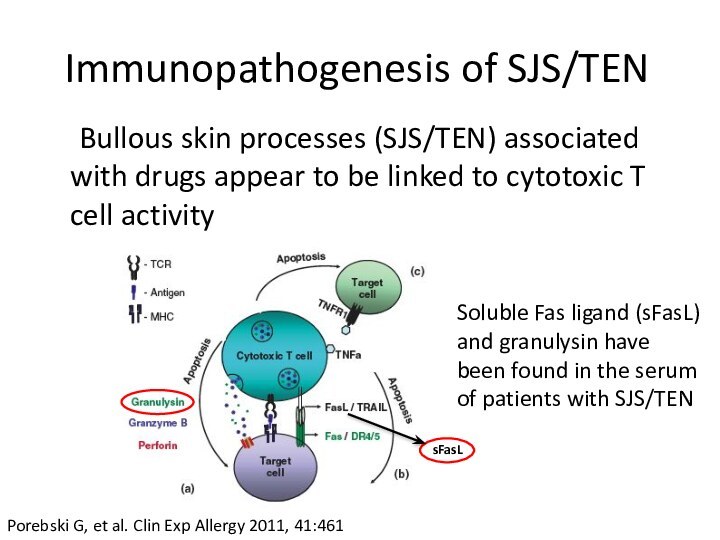
Immunopathogenesis of SJS/TEN
Bullous skin processes (SJS/TEN) associated with
drugs appear to be linked to cytotoxic T cell
activitysFasL
Porebski G, et al. Clin Exp Allergy 2011, 41:461
Soluble Fas ligand (sFasL) and granulysin have been found in the serum of patients with SJS/TEN
Слайд 24
“Real Time” Test to Diagnose SJS/TEN
The serum level
of granulysin is ~100X greater than sFasL in SJS/TEN
making it an attractive targetAn immunochromagraphic test for serum granulysin (>10 ng/mL) predicted SJS/TEN 2-4 days prior to mucocutaneous reuptions
This assay could prove useful in predicting when a drug reaction will lead to SJS/TEN
Fujita Y, et al. J Am Acad Dermatol 2011, 65:65
Слайд 25
In the Future
Multiplex cytokine evaluation following in vitro
culture (e.g. IFN-γ, IL-2, IL-4, IL-5, IL-8, IL-13, IL-17,
etc) may reveal specifics about the type of immune responseNature of drug derived epitopes inducing an immune reaction often are not well understood
Mass spectrometry (MS) has evolved as a powerful tool to evaluate proteomics and metabolomics
MS used to characterize the functional antigens derived from piperacillin (in CF patient serum) with the identification of multiple drug derived haptenic structures bound to albumin (Whitaker P, et al. J Immunol 2011, 187:200)
Слайд 26
Summary in vitro Testing in Drug Allergy
Immediate
drug reactions
Specific IgE testing: safe test but there are
limited numbers of suitable drug conjugates available for testingBAT: promising functional test that requires viable cells and a drug conjugate preparation for activation
Delayed drug reactions
Lymphocyte transformation test (LTT)
Most common research method to determine responsible drug
Issues remaining include: standardization, requirement for viable cells, six day sterile tissue culture period and use of radionuclides
CD69 upregulation may be equivalent to LTT – under study
In vitro cytokine production to drug – under study
Product of cytotoxic cells (granulysin) promising to help dx SJS/TEN prior to mucocutaneous symptoms (further study)
Слайд 27
Conclusions
The clinical story remains the most important starting
point evaluating possible drug allergy
In vitro testing can be
complementary to in vivo testing and is evolving for the evaluation of both immediate and delayed drug allergyThere is currently no single laboratory test that reliably establishes the drug responsible for an immunologically mediated drug reaction