- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Ointments, creams and gels
Содержание
- 2. Contents I. OintmentsII. Compendial requirements for ointmentsIII. CreamsIV. GelsV. Miscellaneous semisolid preparations: pastes and plasters
- 3. VI. Features and use of dermatologic preparationsVII.
- 4. Ointments, creams and gels are semisolid dosage
- 5. Topical preparations are used for both local
- 6. A transdermal product is designed to deliver
- 7. I. Ointments Ointments are semisolid preparations intended
- 8. 1. Ointment bases Ointments bases are classified
- 9. Hydrocarbon bases are also termed oleaginous bases.
- 10. Petrolatum (矿脂) is a purified mixture of semisolid
- 11. White Petrolatum is a purified mixture of
- 12. Yellow ointment is mixture (1000g) of yellow wax
- 13. White ointment This ointment differs from yellow ointment
- 14. 2) Absorption bases Absorption bases are of
- 15. Absorption bases may be used as emollients;are
- 16. Hydrophilic petrolatum Hydrophilic petrolatum, USP has the following
- 17. Lanolinobtained from the wool of sheep; is
- 18. 3) Water-removable basesWater-removable bases are oil-in-water emulsions
- 19. Hydrophilic ointment Hydrophilic ointment has the following formula
- 20. In preparating the ointment, the stearyl alcohol
- 21. 4) Water-soluble basesWater-soluble bases do not contain
- 22. Polyethylene glycol ointmentPolyethylene glycol (PEG) is a
- 23. Selection of the appropriate baseDesired release rate
- 24. Stability of the drug in the ointment
- 25. Preparation of ointments Ointments are prepared by two
- 26. Incorporation By the incorporation method, the components
- 27. A small portion of the powder is
- 28. The drug (the pink powder) is usually the smaller quantity of the two ingredients.
- 29. Add an amount of the ointment that
- 30. Add a second portion of the ointment
- 31. Continue adding until all of the ointment is used. Spatulate after each addition.
- 32. It often is desirable to reduce the
- 33. The amount of levigating agent used should
- 34. Incorporation of liquids: Liquid substances or solutions
- 35. Alcoholic solutions of small volume may be
- 36. Fusion By the fusion method, all or
- 37. On a small scale, the fusion process
- 38. 软膏剂的制备 软膏剂的制备方法分为三种:1. 研和法 2. 熔和法3. 乳化法溶液型或混悬型软膏采用研和法和熔和法乳剂型软膏剂采用乳化法
- 39. 基本制备工艺1. 基质的处理: 一般凡士林、液状石蜡等油脂类基质用前要熔融过滤去除杂质;用于创面的基质要灭菌(150℃, 1小时)。
- 40. 2. 药物的处理:能溶于基质 溶液型不溶性固体药物
- 41. 3.制备方法1)研和法 主要用于半固体油脂性基质的软膏制备此法适用于小量软膏的制备混入基质中的药物常是不溶于基质的
- 42. 方法 先取药物与部分基质或适宜液体研磨成细腻糊状,再递加其余基质研匀,直到制成的软膏涂于皮肤上无颗粒感。 硼酸
- 43. 2)熔和法 主要用于由熔点较高的组分组成、常温下不能均匀混合的软膏基质。此法适用于大量软膏的制备。方法: 先将熔点最高的基质加热熔化,然后将其余基质依熔点高低顺序逐一加入,待全部基质熔化后,再加入药物(能溶者), 搅匀并至冷凝。含不溶性药物粉末的软膏经一般搅拌、混合后尚难制成均匀细腻的产品,可通过研磨机进一步研磨使之细腻均匀。
- 44. 例: 苯甲酸 120g 水杨酸 60g
- 45. 3)乳化法 专门用于制备乳剂型基质软膏剂的方法将处方中油脂性和油溶性组分一并加热熔化,作为油相,保持油相温度在80℃左右;另将水溶性组分溶于水,并加热至与油相相同温度,或略高于油相温度,油、水两相混合,不断搅拌,直至乳化完成并冷凝。
- 46. 乳化法中油、水两相的混合方法:①两相同时掺和,适用于连续的或大批量的操作。②分散相加到连续相中,适用于含小体积分散相的乳剂系统。③连续相加到分散相中,适用于多数乳剂系统,在混合过程中可引起乳剂的转型,从而产生更为细小的分散相粒子 。
- 47. 例: 醋酸曲安缩松 0.25g 二甲基亚砜
- 48. II. Compendial requirements for ointments1) Microbial contentOintments
- 49. Among the antimicrobial preservatives used to inhibit
- 50. 2) Minimum fill (最小装量) The USP’s minimum fill
- 51. 3) Packaging, storage, and labelingIn large-mouth ointment
- 52. In addition to the usual labeling requirements
- 53. 4) Additional standards In addition to the USP
- 54. 软膏剂的质量评价及包装贮存(一)质量检查项目和方法 1.粒度 不得检出大于180μm的粒子。2.装量 照最低装量检查法检查,应符合规定。3. 微生物限度 照微生物限度检查法检查,应符合规定。4.无菌 除另有规定外,软膏剂用于大面积烧伤及严重损伤的皮肤时,照无菌检查法项下的方法检查,应符合规定。
- 55. 5.主药含量 软膏剂采用适宜的溶剂将药物溶解提取,再进行含量测定,测定方法必须考虑和排除基质对提取物含量测定的干扰和影响,测定方法的回收率要符合要求。
- 56. 6.物理性质1)熔点:一般软膏以接近凡士林的熔点为宜。2)粘度与稠度:属牛顿流体的液体石蜡、硅油,测定其粘度可控制质量。软膏剂多属非牛顿流体,除粘度外,常需测定稠度,可用插度计测定,插度计插入样品以0.1mm的深度为一单位,称为插入度(重150g锥体,5s)。一般稠度大的样品插入度小,稠度小的样品插入度大。例如凡土林的插入度在0℃时不得小于100,在37℃时不得大于300;O/W型乳剂基质的插入度(25℃)多在200~300之间较适宜。
- 57. 3)酸碱度:一般控制在pH4.4-8.34)物理外观:色泽均匀一致,质地细腻,无粗糙感,无污物。7.刺激性 考察软膏对皮肤、粘膜有无刺激性或致敏作用。
- 58. 8. 稳定性 可采用加速试验法,将软膏均匀装入密闭容器中填满,分别置恒温箱(39℃±1℃)、室温(25℃±3℃)及冰箱(5℃±2℃ )中至少贮存l-3个月,检查其稠度、酸碱度、性状、均匀性、霉败等现象及药物含量的改变等。乳膏剂应进行耐热、耐寒试验,将供试品分别置于55 ℃恒温6小时及-15℃放置24小时,应无油水分离。一般W/O型乳剂基质耐热性差,油水易分层,O/W型乳剂基质耐寒性差,质地易变粗。
- 59. (二)软膏剂的包装贮存1.包装材料与方法 大量生产均采用软膏管包装,常用有锡管、铝管或塑料管等。2.贮存 包装好的软膏剂一般在常温下避光、密闭条件贮存,温度不宜过高或过低,以免基质分层或药物降解而影响均匀性和疗效。
- 60. III. Creams Pharmaceutical creams are semisolid preparations
- 61. Creams find primary application in topical skin
- 62. IV. Gels Gels are semisolid systems consisting
- 63. Among the gelling agents used are:carbomer 934 (卡波姆), carboxymethylcellulose (羧甲基纤维素),hydroxypropylmethyl-cellulose(羟丙基甲基纤维素),Tragacanth(黄芪胶).
- 64. In addition to the gelling agent and
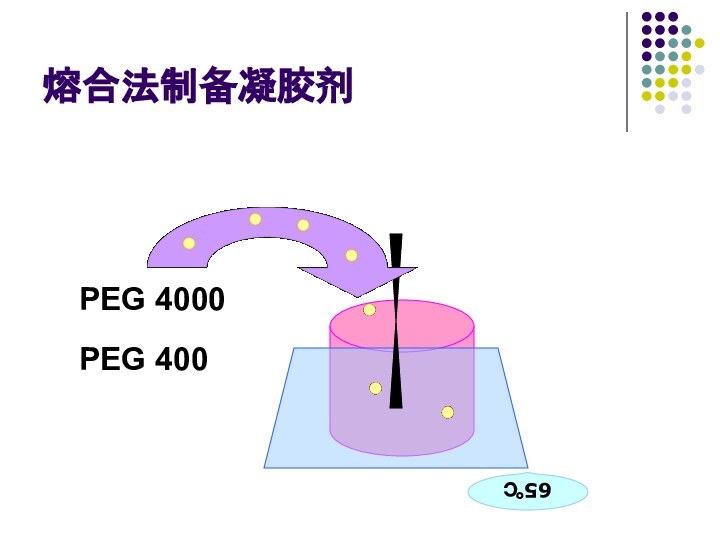
- 65. 熔合法制备凝胶剂PEG 4000PEG 40065℃
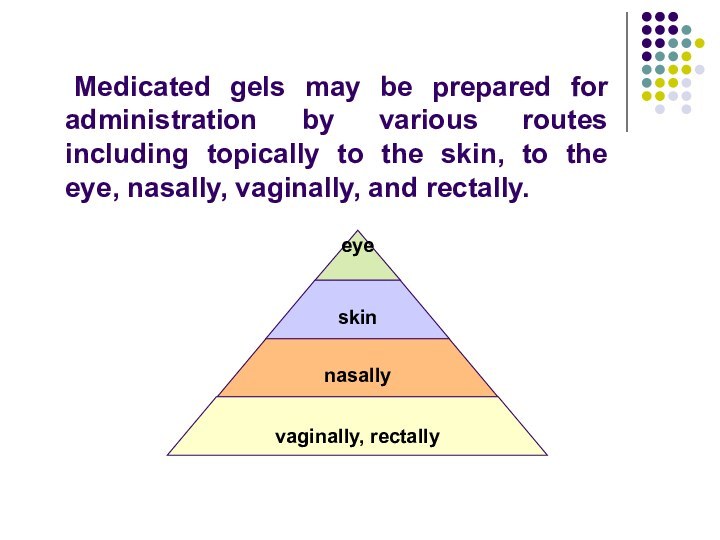
- 66. Medicated gels may be prepared for administration
- 67. V. Miscellaneous semisolid preparations1. Pastes Pastes are
- 68. Pastes are prepared in the same manner
- 69. 2. PlastersPlasters are solid or semisolid adhesive
- 70. 3. glycerogelatins (甘油明胶剂)Glycerogelatins are plastic masses containing
- 71. Adding the medicinal substance mixed with the
- 72. Glycerogelatins are melted before application, cooled to
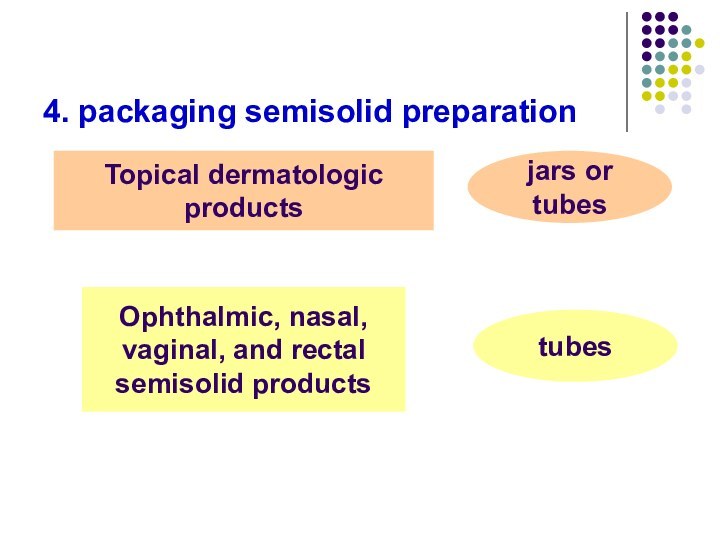
- 73. 4. packaging semisolid preparationTopical dermatologic productsjars or tubesOphthalmic, nasal,vaginal, and rectalsemisolid productstubes
- 74. 1) Filling ointment jarsOintment jars are filled
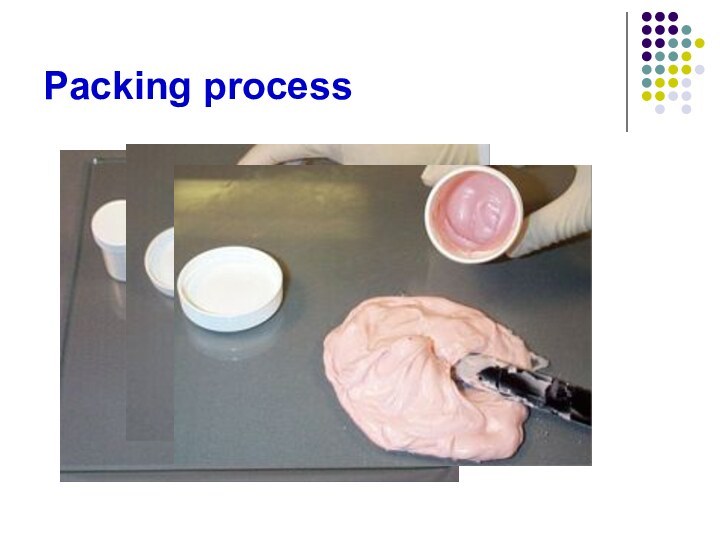
- 75. Packing process
- 76. Packing process
- 77. Packing process
- 78. 2) Filling ointment tubesTubes are filled from
- 81. Industrially, automatic tube-filling, closing, crimping, and labeling
- 82. VI. Features and use of dermatologic preparationsIn
- 83. The skin is divided histologically into the
- 84. Blood capillaries and nerve fibers rise from
- 86. Hair follicles and gland ducts can provide
- 87. The rate of drug movement across the
- 88. For topical products, treatment is based on
- 89. Oleaginous bases provide greater occlusion and emollient
- 90. The pharmacist should be certain that the
- 91. VII. Features and use of ophthalmic ointments
- 92. The cornea is a trilaminate structure with
- 93. Ocular drug penetration is limited due to
- 94. The ointment base selected for an ophthalmic
- 95. The bases in ophthalmic ointments aremixtures of white petrolatum and liquid petrolatum,lanolin,polyethylene glycol,mineral oil.
- 96. In addition to the quality standards for
- 97. VIII. Features and use of nasal ointments
- 98. Drugs introduced into the nasal passage are
- 99. The nasal route of administration is used
- 100. The nasal route holds great promise for
- 101. IX. Features and use of rectal preparationsOintments
- 102. The drugs employed includeastringents 收敛剂 (e.g., zinc
- 103. Substances applied rectally may be absorbed by
- 104. The bases used in anorectal ointments and
- 105. X. Features and use of vaginal preparationsThe
- 106. Among the anti-infective agents used in the various anti-infective products areNystatin (制霉菌素)Clotrimazole (克霉唑)Miconazole (咪康唑)Clindamycin (氯洁霉素)Sulfonamides (磺胺类药物)
- 107. Endometrial atrophy may be treated locally with
- 108. Ointments, creams, and gels for vaginal use are packaged in tubes, vaginal foams in aerosol canisters.
- 109. Скачать презентацию
- 110. Похожие презентации
Contents I. OintmentsII. Compendial requirements for ointmentsIII. CreamsIV. GelsV. Miscellaneous semisolid preparations: pastes and plasters
































































































Слайд 3
VI. Features and use of dermatologic preparations
VII. Features
and use of ophthalmic ointments and gels
VIII. Features and
use of nasal ointments and gelsIX. Features and use of rectal preparations
X. Features and use of vaginal preparations
Слайд 4 Ointments, creams and gels are semisolid dosage forms
intended for topical application.
They may be applied to the
skin, placed onto the surface of the eye, or used nasally, vaginally or rectally. Semisolid Dosage Forms
软膏剂
Ointments
霜剂
Creams
凝胶剂
Gels
Слайд 5 Topical preparations are used for both local and
systemic effects.
A topical dermatological product is designed to deliver
drug into the skin in treating dermal disorders, with the skin as the target organ.Слайд 6 A transdermal product is designed to deliver drugs
through the skin (percutaneous absorption) to the general circulation
for systemic effects, with the skin not being the target organ.Systemic drug absorption should always be considered when using topical products if the patient is pregnant or nursing.
Слайд 7
I. Ointments
Ointments are semisolid preparations intended for
external application to the skin or mucous membranes.
Ointments
may be medicated or nonmedicated.Nonmedicated ointments are used for the physical effects that they provide as protectants, emollients or lubricants.
Слайд 8
1. Ointment bases
Ointments bases are classified by the
USP into four general groups:
hydrocarbon bases
absorption bases
water-removable bases
water-soluble bases
Слайд 9
Hydrocarbon bases are also termed oleaginous bases.
On
application to the skin
1) Hydrocarbon bases
protect against the
escape of moistureemollient effect
occlusive dressings
Слайд 10
Petrolatum (矿脂)
is a purified mixture of semisolid hydrocarbons
obtained from petroleum.
It is an unctuous mass, varying in
color from yellowish to light amber(琥珀色). It melts at temperatures between 38°C and 60 °C and may be used alone or in combination with other agents as an ointment base.
A commercial product is Vaseline.
Слайд 11
White Petrolatum
is a purified mixture of semisolid
hydrocarbons from petroleum that has been wholly or nearly
decolorized.It is used for the same purpose as petrolatum. A commercial product is White Vaseline.
Слайд 12
Yellow ointment
is mixture (1000g) of yellow wax (50g)
and petrolatum (950g).
Yellow wax is the purified wax obtained
from the honeycomb of the bee.The ointment is prepared by melting the yellow wax on a water bath, adding the petrolatum until the mixture is uniform, then cooling with stirring until congealed.
Слайд 13
White ointment
This ointment differs from yellow ointment by
substituting white wax and white petrolatum in the formula.
Слайд 14
2) Absorption bases
Absorption bases are of two
types:
Those that permit the incorporation of aqueous solutions resulting
in the formation of water-in-oil emulsions (e.g., hydrophilic petrolatum)Those that are water-in-oil emulsions and permit the incorporation of additional quantities of aqueous solutions (e.g., Lanolin)
Слайд 15
Absorption bases
may be used as emollients;
are not
easily removed from the skin with water washing since
the external phase of the emulsion is oleaginous;are useful as pharmaceutical adjuncts to incorporate small volumes of aqueous solutions into hydrocarbon bases.
Слайд 16
Hydrophilic petrolatum
Hydrophilic petrolatum, USP has the following formula
for the preparation of 1000 g:
Cholesterol 30 g
Stearyl alcohol(硬脂醇) 30 g
White
wax 80 gWhite petrolatum 860 g
It is prepared by melting the stearyl alcohol and the white wax on a steam bath, adding the cholesterol with stirring until dissolved, adding the white petrolatum and allowing the mixture to cool while being stirred until congealed(凝结).
Слайд 17
Lanolin
obtained from the wool of sheep;
is a
purified, wax-like substance that has been cleaned, deodorized, and
decolorized.It contains not more than 0.25% water.
Additional water may be incorporated into lanolin by mixing.
Слайд 18
3) Water-removable bases
Water-removable bases are oil-in-water emulsions resembling
creams in appearance.
Because the external phase of the
emulsion is aqueous, they are easily washed from skin and are often called ‘water washable’ bases. They may be diluted with water or aqueous solutions.
Слайд 19
Hydrophilic ointment
Hydrophilic ointment has the following formula for
the preparation of about 1000 g:
Methylparaben 0.25g
Propylparaben 0.15g
Sodium lauryl sulfate(月桂醇硫酸钠) 10g
Propylene glycol(丙二醇) 120g
Stearyl
alcohol 250gWhite petrolatum 250g
Purified water 370g
Слайд 20 In preparating the ointment, the stearyl alcohol and
white petrolatum are melted together at about 75°C. The
other agents, dissolved in the purified water, are added with stirring until the mixture congeals.
Слайд 21
4) Water-soluble bases
Water-soluble bases do not contain oleaginous
components.
They are completely water-washable and often referred to
as ‘greaseless’(无脂物). Because they soften greatly with the addition of water, large amounts of aqueous solutions are not effectively incorporated into these bases.
They mostly are used for the incorporation of solid substances.
Слайд 22
Polyethylene glycol ointment
Polyethylene glycol (PEG) is a polymer
of ethylene oxide and water represented by the formula:
H(OCH2CH2)nOH in which n represents the average number of oxyethylene groups.PEGs having average molecular weights below 600 are clear, colorless liquids; those with molecular weights above 1000 are wax-like white materials; those with molecular weights in between are semisolids.
Слайд 23
Selection of the appropriate base
Desired release rate of
the drug substance from the ointment base;
Desirability for topical
or percutaneous drug absorption;Desirability of occusion of moisture from the skin;
Слайд 24
Stability of the drug in the ointment base;
Effect
of the drug on the consistency or other features
of the ointment baseThe desire for a base that is easily removed by washing with water.
Слайд 25
Preparation of ointments
Ointments are prepared by two general
methods:
Incorporation (加入法)
Fusion(融合法)
The method used depends primarily on the
nature of the ingredients.
Слайд 26
Incorporation
By the incorporation method, the components are
mixed until a uniform preparation is attained.
Incorporation of
solids: The ointment base is placed on one side of the working surface and the powdered components, previously reduced to fine powders and thoroughly blended in a mortar, on the other side.
Слайд 27 A small portion of the powder is mixed
with a portion of the base until uniform.
The
process is continued until all portions of the powder and base are combined and thoroughly and uniformly blended.
Слайд 29
Add an amount of the ointment that is
approximately equal in size to the drug.
Spatulate the mixture.
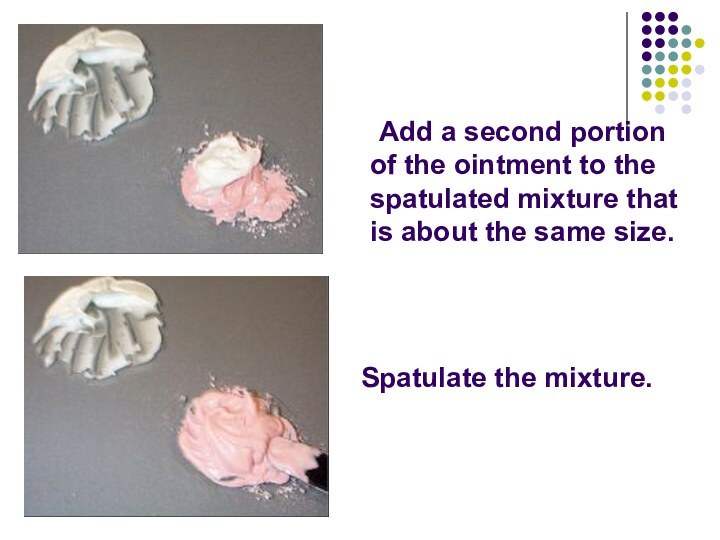
Слайд 30
Add a second portion of the ointment to
the spatulated mixture that is about the same size.
Spatulate
the mixture.Слайд 32 It often is desirable to reduce the particle
size of a powder or crystalline material before incorporation
into the ointment base so that the final product will not be gritty.This may be done by levigating or mixing the solid material in a vehicle in which it is insoluble to make a smooth dispersion.
Слайд 33 The amount of levigating agent used should be
about equal in volume to the solid material.
A
mortar and pestle is used for levigation. This allows both reduction of particle size and the dispersion of the substance in the vehicle. After levigation, the dispersion is incorporated into the ointment base by spatulation or with the mortar and pestle until the product is uniform.
Слайд 34
Incorporation of liquids:
Liquid substances or solutions of
drugs are added to an ointment only after due
consideration of an ointment base’s capacity to accept the volume required.When it is necessary to add an aqueous preparation to a hydrophobic base, the solution first may be incorporated into a minimum amount of a hydrophilic base and then that mixture added to the hydrophobic base.
Слайд 35 Alcoholic solutions of small volume may be added
quite well to oleaginous vehicles or emulsion bases.
On a
large scale, roller mills force coarsely formed ointments through stainless steel rollers to produce ointments that are uniform in composition and smooth in texture.
Слайд 36
Fusion
By the fusion method, all or some
of the components of an ointment are combined by
being melted together and cooled with constant stirring until congealed.Medicated ointments and ointment bases containing components as beeswax, paraffin, stearyl alcohol, and high molecular weight polyethylene glycols, which do not lend themselves well to mixture by incorporation, are prepared by fusion.
Слайд 37 On a small scale, the fusion process may
be conducted in a porcelain dish(陶瓷盘) or glass beaker.
On
a large scale, it is carried out in large steam-jacketed kettles(蒸气夹层加热容器). Once congealed, the ointment may be passed through an ointment mill (in large-scale manufacture) or rubbed with a spatula or in a mortar (in small-scale preparation) to ensure a uniform texture.
Слайд 40
2. 药物的处理:
能溶于基质 溶液型
不溶性固体药物
磨成细粉,过100~120目筛,与基质混匀。
可溶性药物 溶于适宜溶剂 基质混匀。
半固体粘稠药物,煤焦油(表面活性剂),固体浸膏(乙醇)
挥发性共熔组分
先成共熔物 冷至40℃以下的基质混匀,也可溶于溶剂后与适宜基质混匀。
Слайд 42
方法
先取药物与部分基质或适宜液体研磨成细腻糊状,再递加其余基质研匀,直到制成的软膏涂于皮肤上无颗粒感。
硼酸 100g
主药(过9号筛)
凡士林 100g
基质制成 1000g
制法:取硼酸加少量凡士林研匀后,缓缓加入剩余的基质,继续研磨,直至涂抹到皮肤表面无粗糙感。
Слайд 43
2)熔和法
主要用于由熔点较高的组分组成、常温下不能均匀混合的软膏基质。
此法适用于大量软膏的制备。
方法:
先将熔点最高的基质加热熔化,然后将其余基质依熔点高低顺序逐一加入,待全部基质熔化后,再加入药物(能溶者), 搅匀并至冷凝。含不溶性药物粉末的软膏经一般搅拌、混合后尚难制成均匀细腻的产品,可通过研磨机进一步研磨使之细腻均匀。
Слайд 44
例: 苯甲酸 120g
水杨酸 60g
液体石蜡
100g
羊毛脂 100g
石 蜡
适量凡士林 加至1000g
取苯甲酸、水杨酸细粉加液体石蜡研成糊状;另将羊毛脂、凡士林、石蜡加热熔化,经细布过滤,待温度降至60℃以下时加入上述药物,搅匀至冷凝。
抗霉菌及角质剥脱作用,用于手足癣及体股癣。
Слайд 45
3)乳化法
专门用于制备乳剂型基质软膏剂的方法
将处方中油脂性和油溶性组分一并加热熔化,作为油相,保持油相温度在80℃左右;另将水溶性组分溶于水,并加热至与油相相同温度,或略高于油相温度,油、水两相混合,不断搅拌,直至乳化完成并冷凝。
Слайд 46
乳化法中油、水两相的混合方法:
①两相同时掺和,适用于连续的或大批量的操作。
②分散相加到连续相中,适用于含小体积分散相的乳剂系统。
③连续相加到分散相中,适用于多数乳剂系统,在混合过程中可引起乳剂的转型,从而产生更为细小的分散相粒子 。
Слайд 47
例: 醋酸曲安缩松 0.25g 二甲基亚砜 15g
尿 素 100g
硬脂酸 120g单硬脂酸甘油酯 35g 白凡士林 50g
液状石蜡 100g 甘 油 50g
对羟基苯甲酸乙酯 1.5g 三乙醇胺 4g
蒸馏水加至 1000g
取硬脂酸、单硬脂酸甘油酯、白凡士林 、液状石蜡加热熔化,混匀,经细布
滤过,保温80℃左右。另将尿素、对羟基苯甲酸乙酯、甘油、三乙醇胺溶于
热蒸馏水中,并于80℃左右缓缓加入到油相中,不断搅拌制成乳剂基质。将
醋酸曲安缩松溶于二甲基亚砜后,加至乳膏基质中混匀,即得。
药物不溶于水及基质,用二甲基亚砜溶解后加至基质中有利于小剂量药物以
细小颗粒分散,从而提高疗效。皮质激素类药物需透入表皮后才能发挥其局
部抗炎作用,尿素能促进药物的透皮,可提高疗效,但尿素易受热分解,应
控制水相温度不超过85℃。本品用于过敏性皮肤病、皮炎、湿疹及银屑病。
Слайд 48
II. Compendial requirements for ointments
1) Microbial content
Ointments must
meet acceptable standards for microbial content and preparations which
are prone to microbial growth must be preserved with antimicrobial preservatives.Слайд 49 Among the antimicrobial preservatives used to inhibit microbial
growth in topical preparations are:
methylparaben,
propylparaben,
phenols,
benzoic
acid, sorbic acid,
quaternary ammonium salts.
Слайд 50
2) Minimum fill (最小装量)
The USP’s minimum fill test
involves the determination of the net weight or volume
of the contents of filled containers to assure proper contents compared with the labeled amount.
Слайд 51
3) Packaging, storage, and labeling
In large-mouth ointment jars
or in metal or plastic tubes;
In well-closed containers to
protect against contamination and in a cool place to protect against product separation due to heat;Слайд 52 In addition to the usual labeling requirements for
pharmaceutical products, the USP directs that the labeling for
certain ointments and creams include the type of base used (e.g., water-soluble or water-insoluble).
Слайд 53
4) Additional standards
In addition to the USP requirements,
manufacturers often examine semisolid preparations
for viscosity
for in
vitro drug release to ensure intralot and lot-to-lot uniformity.
Слайд 54
软膏剂的质量评价及包装贮存
(一)质量检查项目和方法
1.粒度 不得检出大于180μm的粒子。
2.装量 照最低装量检查法检查,应符合规定。
3. 微生物限度 照微生物限度检查法检查,应符合规定。
4.无菌
除另有规定外,软膏剂用于大面积烧伤及严重损伤的皮肤时,照无菌检查法项下的方法检查,应符合规定。
Слайд 56
6.物理性质
1)熔点:一般软膏以接近凡士林的熔点为宜。
2)粘度与稠度:属牛顿流体的液体石蜡、硅油,测定其粘度可控制质量。软膏剂多属非牛顿流体,除粘度外,常需测定稠度,可用插度计测定,插度计插入样品以0.1mm的深度为一单位,称为插入度(重150g锥体,5s)。一般稠度大的样品插入度小,稠度小的样品插入度大。例如凡土林的插入度在0℃时不得小于100,在37℃时不得大于300;O/W型乳剂基质的插入度(25℃)多在200~300之间较适宜。
Слайд 58
8. 稳定性
可采用加速试验法,将软膏均匀装入密闭容器中填满,分别置恒温箱(39℃±1℃)、室温(25℃±3℃)及冰箱(5℃±2℃ )中至少贮存l-3个月,检查其稠度、酸碱度、性状、均匀性、霉败等现象及药物含量的改变等。
乳膏剂应进行耐热、耐寒试验,将供试品分别置于55 ℃恒温6小时及-15℃放置24小时,应无油水分离。一般W/O型乳剂基质耐热性差,油水易分层,O/W型乳剂基质耐寒性差,质地易变粗。
Слайд 59
(二)软膏剂的包装贮存
1.包装材料与方法
大量生产均采用软膏管包装,常用有锡管、铝管或塑料管等。
2.贮存
包装好的软膏剂一般在常温下避光、密闭条件贮存,温度不宜过高或过低,以免基质分层或药物降解而影响均匀性和疗效。
Слайд 60
III. Creams
Pharmaceutical creams
are semisolid preparations containing
one or more medical agents dissolved or dispersed in
either an oil-in-water emulsion or in another type of water-washable base.Слайд 61 Creams find primary application in topical skin products
and in products used rectally and vaginally.
Many patients and
physicians prefer creams to ointments because they are easier to spread and remove than many ointments.
Слайд 62
IV. Gels
Gels are semisolid systems consisting of
dispersions of small or large molecules in an aqueous
liquid vehicle rendered jelly-like through the addition of a gelling agent.
Слайд 63
Among the gelling agents used are:
carbomer 934 (卡波姆),
carboxymethylcellulose (羧甲基纤维素),
hydroxypropylmethyl-cellulose(羟丙基甲基纤维素),
Tragacanth(黄芪胶).
Слайд 64 In addition to the gelling agent and water,
gels may be formulated to contain a drug substance,
co-solvents as alcohol and/or propylene glycol, antimicrobial preservatives as methylparaben and propylparaben or chlorhexidine gluconate(葡萄糖酸洗必泰), and stabilizers as edetate disodium(依地酸二钠).Gelling agent
Water
Preservatives
Stabilizers
Слайд 66 Medicated gels may be prepared for administration by
various routes including topically to the skin, to the
eye, nasally, vaginally, and rectally.
Слайд 67
V. Miscellaneous semisolid preparations
1. Pastes
Pastes are semisolid
preparations intended for application to the skin;
They generally contain
a larger proportion of solid material than ointments and therefore are stiffer.Слайд 68 Pastes are prepared in the same manner as
ointments.
Because of the stiffness of pastes, they remain in
place after application and are effectively employed to absorb serous secretions.Because of their stiffness and impenetrability, pastes are not suited for application to hairy parts of the body.
Слайд 69
2. Plasters
Plasters are solid or semisolid adhesive masses
spread upon a backing material of paper, fabic(布), moleskin(兽皮)
or plastic.Plasters are applied to the skin to provide prolonged contact at the site.
Слайд 70
3. glycerogelatins (甘油明胶剂)
Glycerogelatins are plastic masses containing gelatin
(15%), glycerin (40%), water (35%), and an added medical
substance (10%) as zinc oxide.They are prepared by
First softening the gelatin in the water for about 10 minutes, heating on a steam bath until the gelatin is dissolved,
Слайд 71
Adding the medicinal substance mixed with the glycerin,
Allowing
the mixture to cool with stirring until congealed.
Glycerogelatin are
applied to the skin for long-term residence.Слайд 72 Glycerogelatins are melted before application, cooled to slightly
above body temperature, and applied to the affected area
with a fine brush.Following application, the glycerogelatin hardens, is usually covered with a bandage, and is allowed to remain in place for weeks.
The most recent official glycerogelatin was zinc gelatin, used in the treatment of varicose ulcers.
Слайд 73
4. packaging semisolid preparation
Topical dermatologic
products
jars or tubes
Ophthalmic,
nasal,
vaginal, and rectal
semisolid products
tubes
Слайд 74
1) Filling ointment jars
Ointment jars are filled on
a small scale in the pharmacy by carefully transferring
the weighed amount of ointment into the jar with a spatula.The ointment is packed on the bottom along the sides of the jar, avoiding entrapment of air.
In large-scale manufacture of ointments, pressure fillers force the specified amount of ointment into the jars.
Слайд 78
2) Filling ointment tubes
Tubes are filled from the
open back end of the tube, opposite from the
cap end.On a small scale, the tube may be filled manually or with a small scale filling manually.
After filling, the tube is closed and sealed.
Слайд 81 Industrially, automatic tube-filling, closing, crimping, and labeling machines
are used for the large-scale packaging of semisolid pharmaceuticals.
Depending
on the model, machines are available which have the capacity to fill from about 1000 to up to 6000 tubes per hour.
Слайд 82
VI. Features and use of dermatologic preparations
In treating
skin diseases, the drug in a medicated application should
penetrate and be retained in the skin for a period of time.Drug penetration into skin depends on a number of factors including
the physicochemical properties of the medicinal substance,
the characteristics of the pharmaceutical vehicle,
the condition of skin itself.
Слайд 83
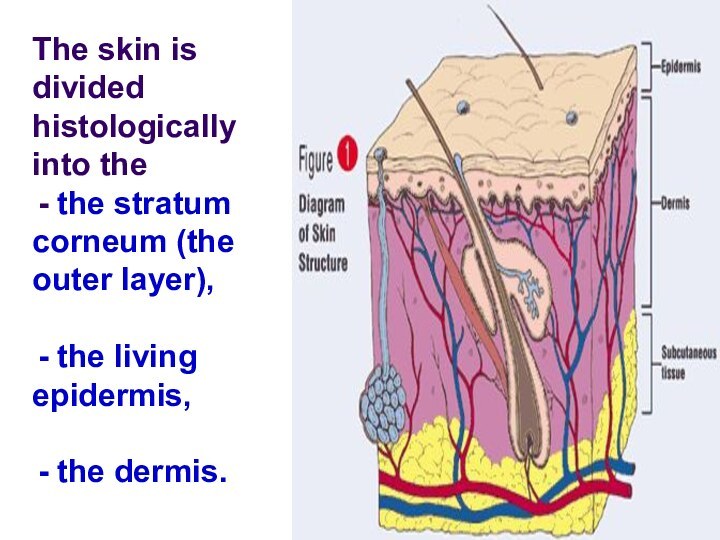
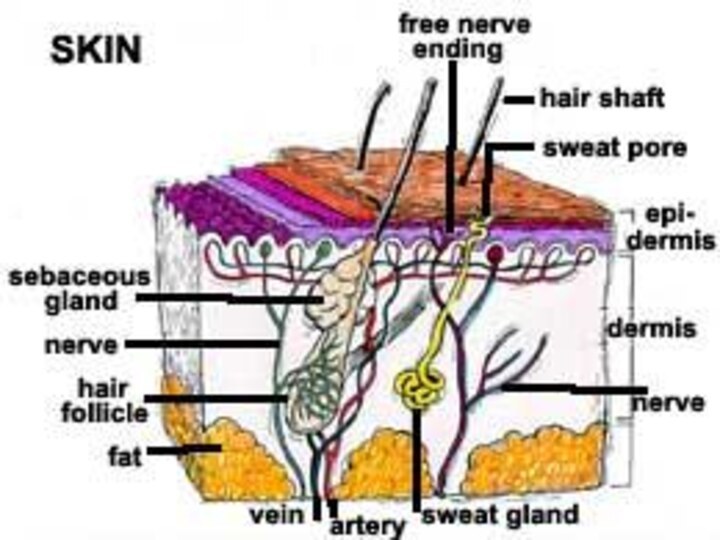
The skin is divided histologically into the
the
stratum corneum (the outer layer),
the living epidermis,
the
dermis.Слайд 84 Blood capillaries and nerve fibers rise from the
subcutaneous fat tissue into the dermis and subcutaneous layers
rise to the skin’s surface.Sebaceous glands, sweat glands, and hair follicles originating in the dermis and subcutaneous layers rise to the skin’s surface.
Слайд 86 Hair follicles and gland ducts can provide entry
for drug molecules, but because their relative surface area
is so minute compared to the total epidermis they are minor factors in drug absorption.The stratum corneum, being keratinized tissue, behaves as a semipermeable artificial membrane, and drug molecules can penetrate by passive diffusion.
Слайд 87 The rate of drug movement across the skin
layer depends on
the drug concentration in the vehicle,
its aqueous
solubility,the oil/water partition coefficient between the stratum corneum and the product’s vehicle.
Substances that possess both aqueous and lipid solubility charateristics are good candidates for diffusion through the stratum corneum.
Слайд 88 For topical products, treatment is based on qualitative
measures with clinical efficacy often varying between patients and
products.Differences in emollient and occlusive effects and ease of application and removal between products is a factor of the base used and product type.
Слайд 89 Oleaginous bases provide greater occlusion and emollient effects
than do hydrophilic or water-washable bases.
Pastes offer even greater
occlusion and are more effective than ointments at absorbing serous discharge.Creams, usually oil-in-water emulsions, spread more easily than ointments and are easier for the patient to remove.
Water-soluble bases are nongreasy and are applied and removed easily.
Слайд 90 The pharmacist should be certain that the patient
understands
the proper method of administration,
frequency and duration
of use,special warnings,
therapeutic goals,
signs of adverse response,
allergic sensitivity, etc.
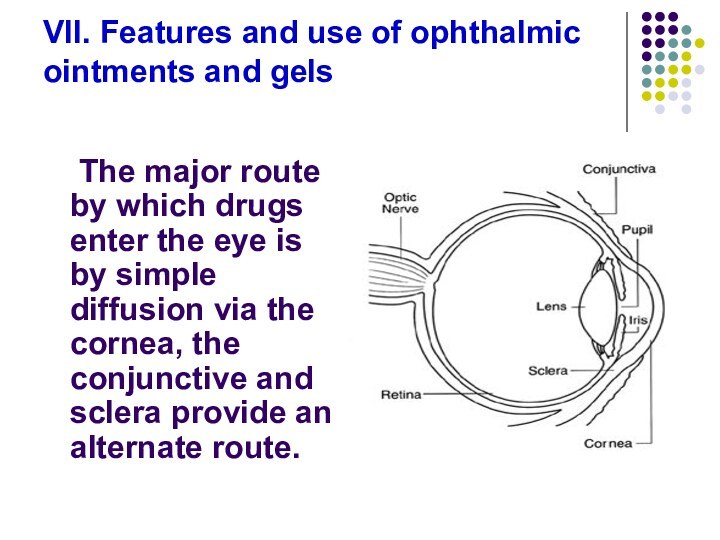
Слайд 91 VII. Features and use of ophthalmic ointments and
gels
The major route by which drugs enter the eye
is by simple diffusion via the cornea, the conjunctive and sclera provide an alternate route.
Слайд 92
The cornea is a trilaminate structure with
a
lipophilic epithelial layer,
a hydrophilic stromal layer,
an less lipophilic endothelial
layer on the inside.Lipophilic drugs are more capable of penetration than hydrophilic compounds.
Слайд 93 Ocular drug penetration is limited due to the
short residence time that ophthalmic preparations have on the
surface of the eye because oftheir rapid removal by tearing and other natural mechanisms,
the small surface area of the cornea for drug absorption,
the cornea’s natural resistance to drug penetration.
Слайд 94
The ointment base selected for an
ophthalmic ointment
must
be non-irritating to the eye,
must permit the diffusion of
the medicinal substance throughout the secretions bathing the eye,should have a softening point close to body temperature.
Слайд 95
The bases in ophthalmic ointments are
mixtures of white
petrolatum and liquid petrolatum,
lanolin,
polyethylene glycol,
mineral oil.
Слайд 96 In addition to the quality standards for ointments,
ophthalmic ointments also must meet
the USP Sterility Tests
the
test Metal Particles Слайд 97 VIII. Features and use of nasal ointments and
gels
The nose is a respiratory organ which is a
passage-way for air to the lungs.Its surface is coated with a continuous thin layer of mucous.
The mucous contains lysozyme, glycoproteins and immunoglobulins.
Слайд 98 Drugs introduced into the nasal passage are primarily
for localized effects on the mucous membranes and underlying
tissues.Drug absorption to the general circulation does occur through the rich blood supply feeding the nasal lining.
Слайд 99 The nasal route of administration is used for
the systemic absorption of a number of drugs including
butorphanol
tartrate(酒石酸布托啡诺) analgesiccyanocobalamin(维生素B12) hematopoietic (造血剂)
narfaralin acetates, endometriosis (子宫内膜异位)
nicotine
Слайд 100 The nasal route holds great promise for the
administration of insulin, vaccines and a number of other
polypeptides and proteins.
Слайд 101
IX. Features and use of rectal preparations
Ointments and
creams are used for topical application to the perianal
area and for insertion within the anal canal.They largely are used to treat local conditions of
anorectal pruritus (瘙痒症)
inflammation
the pain and discomfort
associated with hemorrhoids (痔疮).
Слайд 102
The drugs employed include
astringents 收敛剂 (e.g., zinc oxide)
protectants
and lubricants (e.g., cocoa butter, lanolin)
local anesthetics (e.g., pramoxine
HCl,盐酸普莫卡因),Antipruritics (抗瘙痒)
anti-inflammatory agents (e.g., hydrocortisone)
Слайд 103 Substances applied rectally may be absorbed by diffusion
into the general circulation via the network of three
hemorrhoidal arteries and accompanying veins in the anal canal.The rectal route is used for the systemic absorption of therapeutic levels of certain drugs (e.g., prochlorperazine 氯吡嗪) when oral route is unsatisfactory, as in conditions of vomiting.
Слайд 104 The bases used in anorectal ointments and creams
include
combinations of polyethylene glycol 300 and 3350,
emulsion cream bases
utilizing cetyl alcohol (十六醇)and cetyl esters (十六酯) wax,white petrolatum,
mineral oil.
Слайд 105
X. Features and use of vaginal preparations
The vaginal
surface is lined with squamous(皱纹状)epithelium cells and mucous produced
from various underlying glands.Topical products are used to treat
Vulvovaginal (外阴) infections
Vaginitis (阴道炎)
conditions of endometrial atrophy (子宫内膜萎缩症)
for contraception with spermatocidal agents
Слайд 106 Among the anti-infective agents used in the various
anti-infective products are
Nystatin (制霉菌素)
Clotrimazole (克霉唑)
Miconazole (咪康唑)
Clindamycin (氯洁霉素)
Sulfonamides (磺胺类药物)
Слайд 107 Endometrial atrophy may be treated locally with the
hormonal substances dienestrol(双烯雌酚) and progesterone(黄体酮) which are used to
restore the vaginal mucosa to its normal state.Contraceptive preparations containing spermicidal agents as nonoxynol-9 (壬苯醇醚)and octoxynol (辛苯聚糖)are used alone or in combination with a cervical diaphragm(避孕环)。