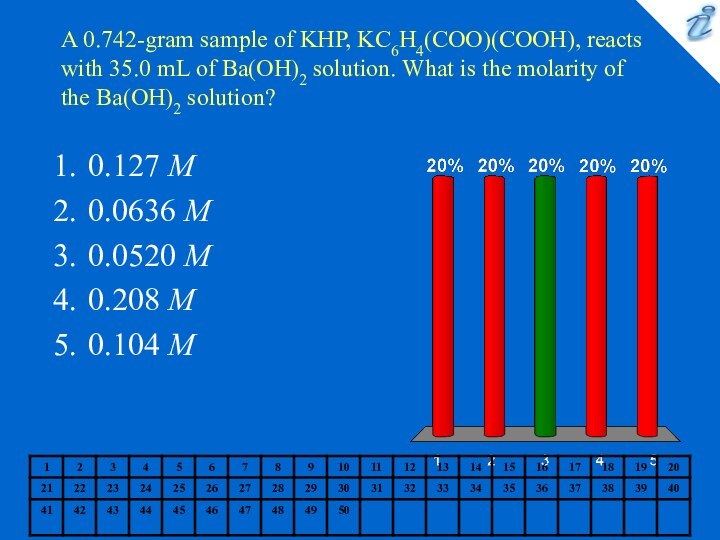
35.0 mL of Ba(OH)2 solution. What is the molarity
of the Ba(OH)2 solution?0.127 M
0.0636 M
0.0520 M
0.208 M
0.104 M
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Email: Нажмите что бы посмотреть
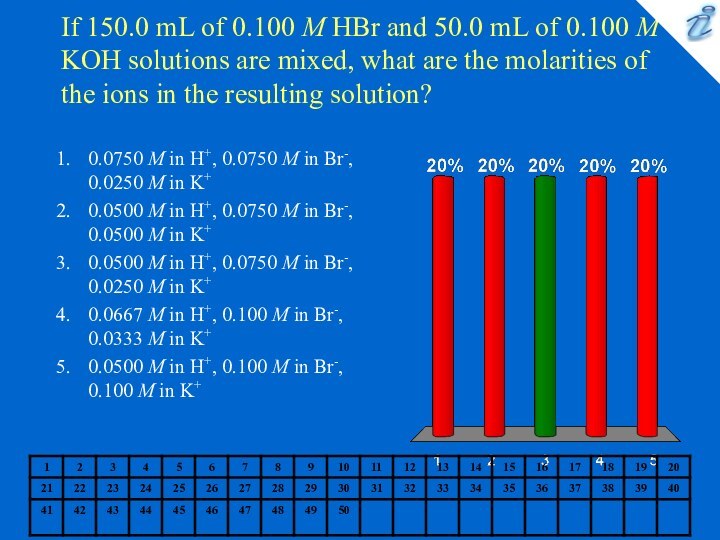
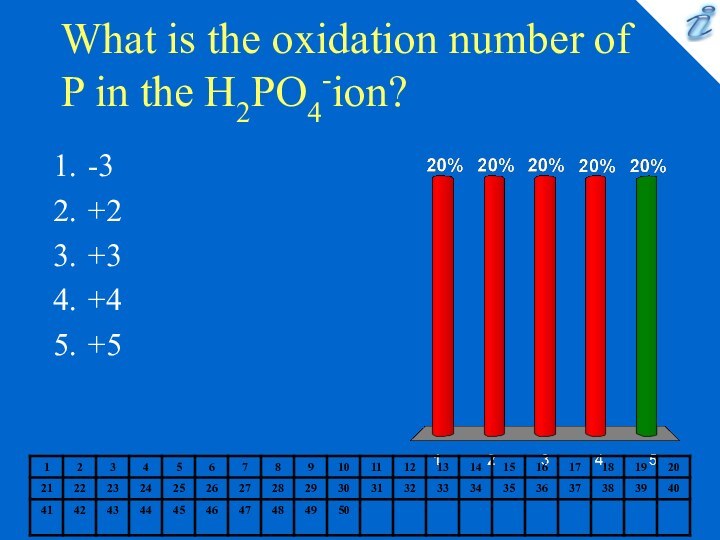
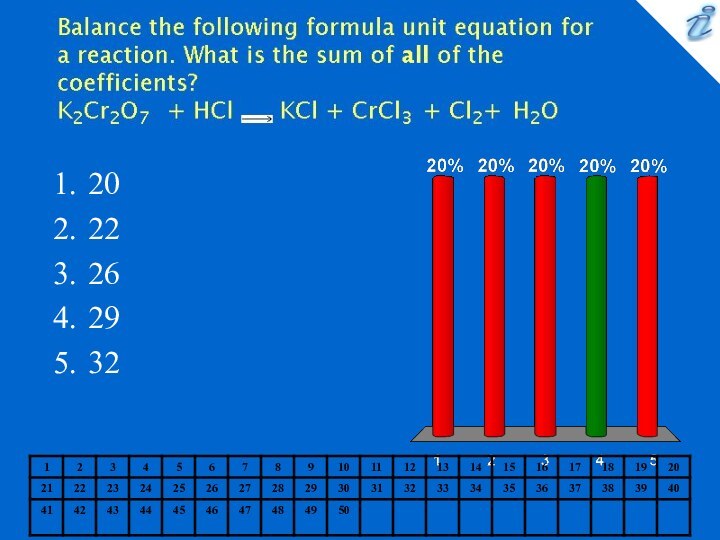
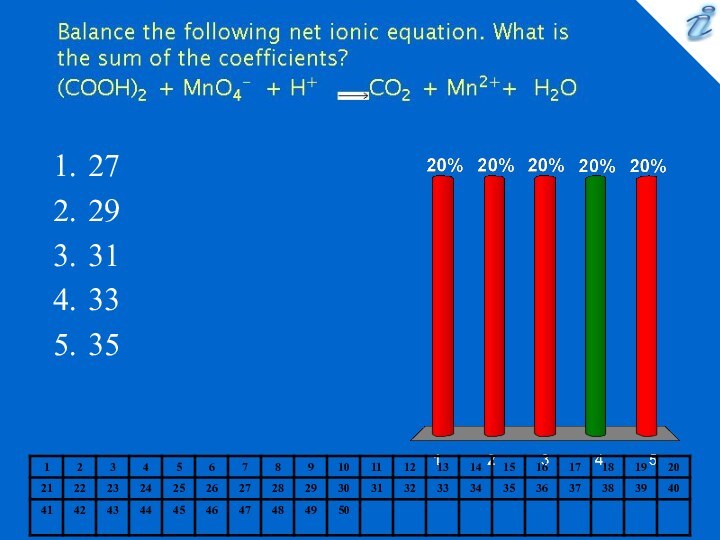
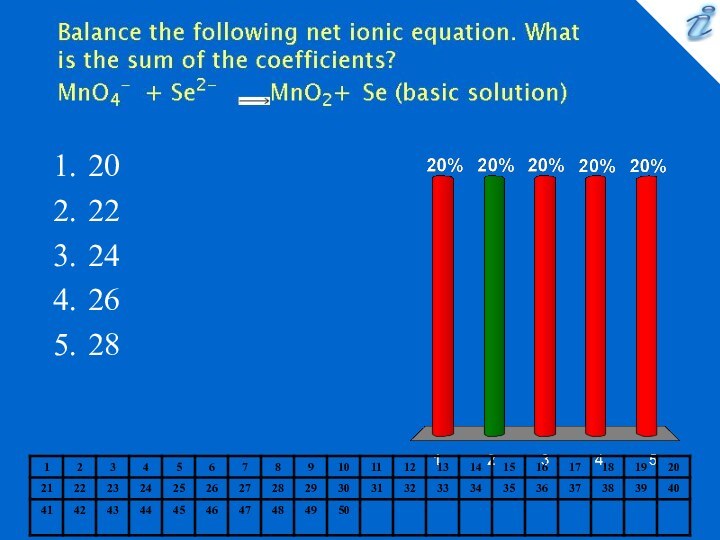
0.127 M
0.0636 M
0.0520 M
0.208 M
0.104 M
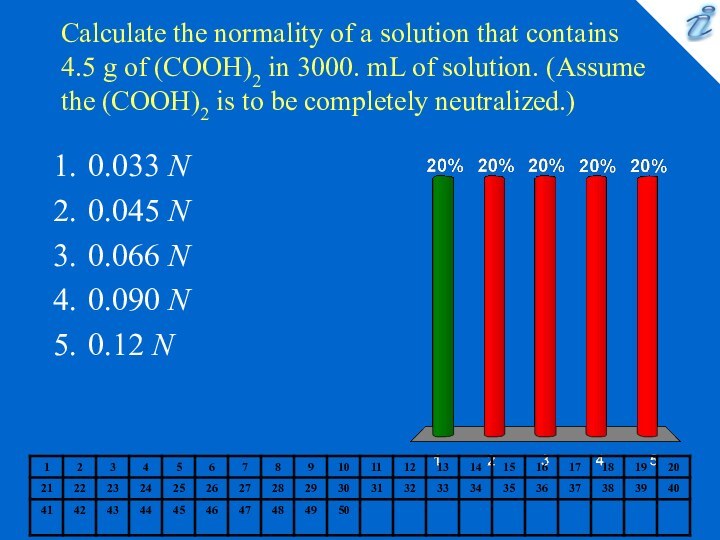
0.033 N
0.045 N
0.066 N
0.090 N
0.12 N
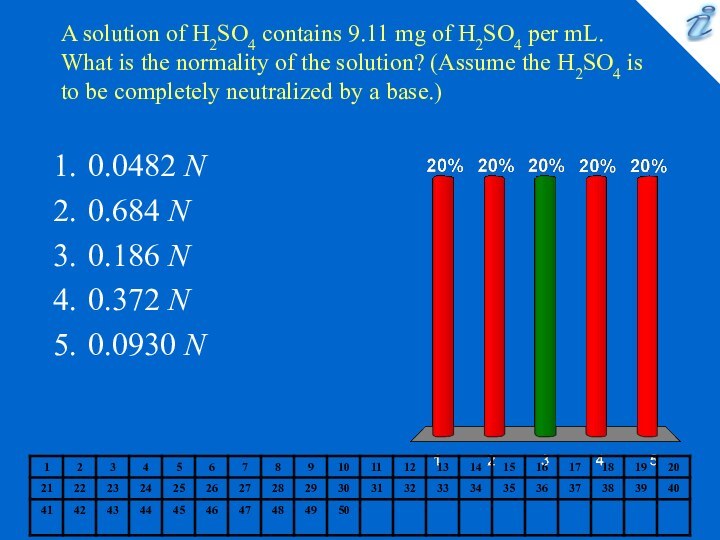
0.0482 N
0.684 N
0.186 N
0.372 N
0.0930 N
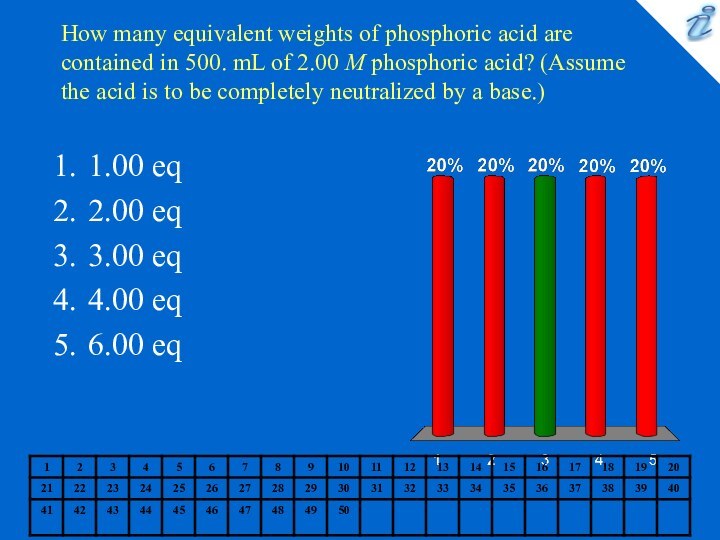
1.00 eq
2.00 eq
3.00 eq
4.00 eq
6.00 eq
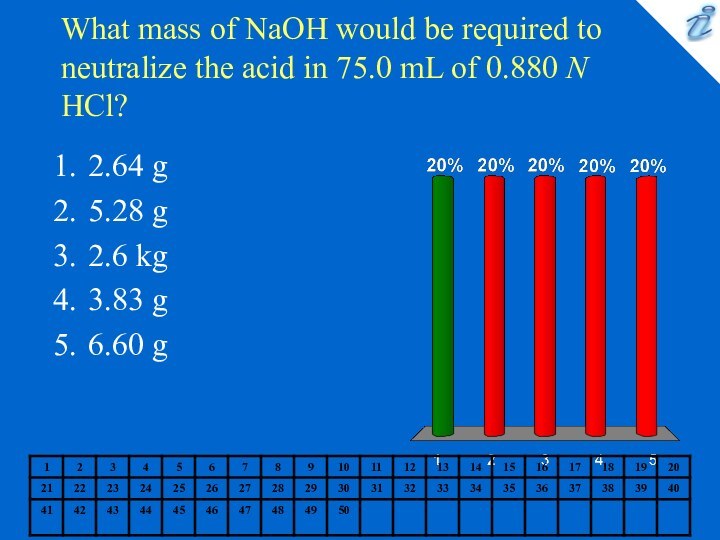
2.64 g
5.28 g
2.6 kg
3.83 g
6.60 g
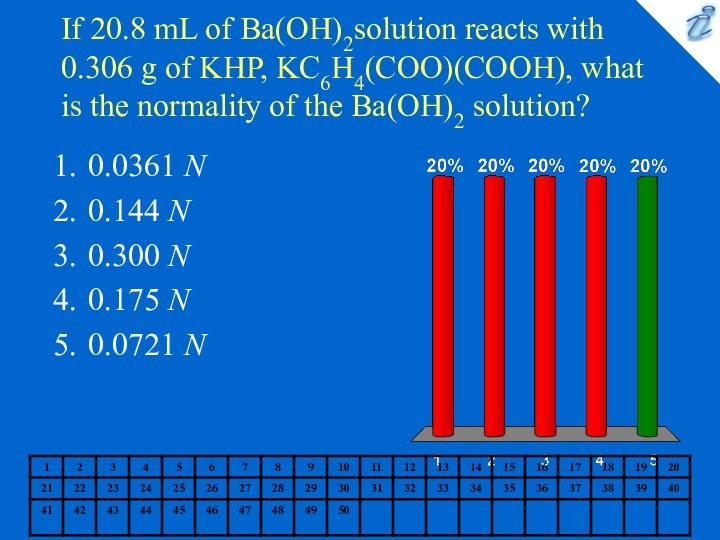
0.0361 N
0.144 N
0.300 N
0.175 N
0.0721 N