Слайд 2
IRON METABOLISM
Iron has the capacity to accept and
donate electrons: Fe2+⮀Fe3+, this capability makes it useful component
of cytochromes, O2-binding molecules.
Iron can damage tissues by producing free radicals that attack cellular membranes, proteins, DNA.
Слайд 3
Proteins of Iron Transport, Uptake and Storage
Transferrin –
a transport protein, carries iron in the plasma and
ECF to supply tissue needs.
Transferrin receptor – a glycoprotein on cell membranes, binds the transferrin-iron complex and is internalized as a vesicle.
Ferritin – iron storage protein.
Слайд 4
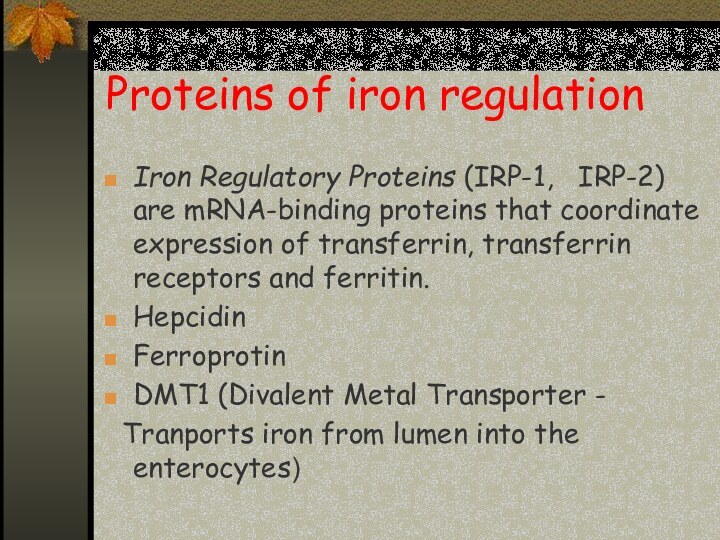
Proteins of iron regulation
Iron Regulatory Proteins (IRP-1,
IRP-2) are mRNA-binding proteins that coordinate expression of transferrin,
transferrin receptors and ferritin.
Hepcidin
Ferroprotin
DMT1 (Divalent Metal Transporter -
Tranports iron from lumen into the enterocytes)
Слайд 5
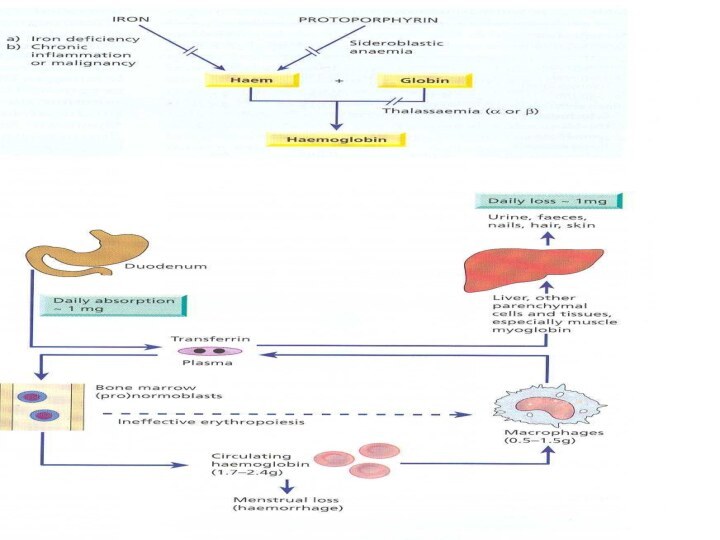
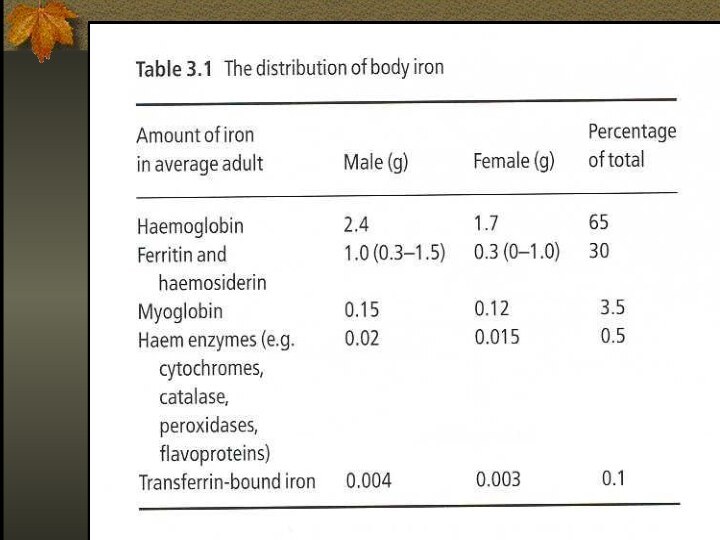
Iron Metabolism
Adult man normally have 35-45mg/kg iron, women
have less.
2/3 of body iron is in haemoglobin in
erythron (RBC precursors in the marrow + RBC in blood)
Most of the remaining iron is found in hepatocytes and reticuloendothelial macrophages which serve as depots
Слайд 8
IRON METABOLISM
Dietary Iron:
Iron is essential
element and must be
precisely regulated.
On the
lumen side of small intestine iron is reduced from its ferric form (Fe3+) to ferrous form (Fe2+).
Ferrous iron is then transported in enterocytes by DMT1 (divalent metal transporter).
Слайд 9
Regulation of Iron Absorption
Humans have no physiologic way
for iron excretion and regulation of absorption is crucial.
The
absorption takes place at gastrodeuodenal junction in acid environment.
There is no role for transferrin in intestinal absorption of iron.
Hepsidin, Ferriprotin, DMT-1
Слайд 10
TRANSPORT PROTEINS
DMT1 (Divalent Metal Transporter 1)
(Tranports from lumen into the enterocytes)
FERROPORTIN1
(Transports
from enterocytes to circulation)
Слайд 11
Hepicidin, Primary regulator
Increased expression of hepicidin leads
to
Decrease iron absorption and release.
Mutation :Hemochromatosis
Increased expression: Iron deficiency
Hepicidin mRna expression is increased by erythropoetin, hypoxia & inflammation.
Also binds to ferroportin.
Слайд 12
Hepcidin
A 25 amino
acid polypeptide produced by liver cells
An acute phase protein
The
major hormonal regulator of iron homeostasis
Inhibits Fe release from macrophages, intestinal epithelial cells and from placenta
Interaction with transmembrane Fe transporter ferroportin (decrease)
Inflammatory cytokines IL-6, TNF induce hepcidin
Iron deficiency, hypoxia and ineffective erythropoesis Decreased hepcidin
Слайд 13
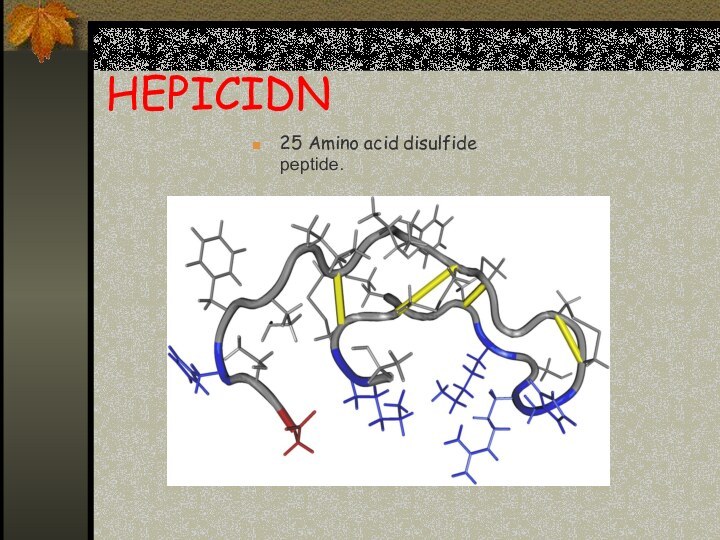
HEPICIDN
25 Amino acid disulfide peptide.
Слайд 14
O
Hepcidin lowers iron absorption in the intestine ,
lowers iron releasing from hepatocytes and macrophages
Serum iron is decreased.
Слайд 15
Ferroportin
The only cellular iron exporter in vertebrates.
Present in macrophages, placenta and the hepatocytes.
Слайд 16
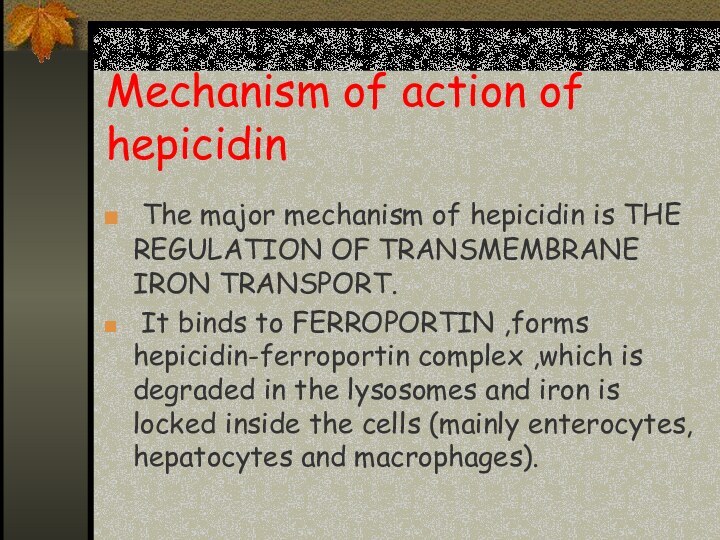
Mechanism of action of hepicidin
The major mechanism
of hepicidin is THE REGULATION OF TRANSMEMBRANE IRON TRANSPORT.
It binds to FERROPORTIN ,forms hepicidin-ferroportin complex ,which is degraded in the lysosomes and iron is locked inside the cells (mainly enterocytes, hepatocytes and macrophages).
Слайд 17
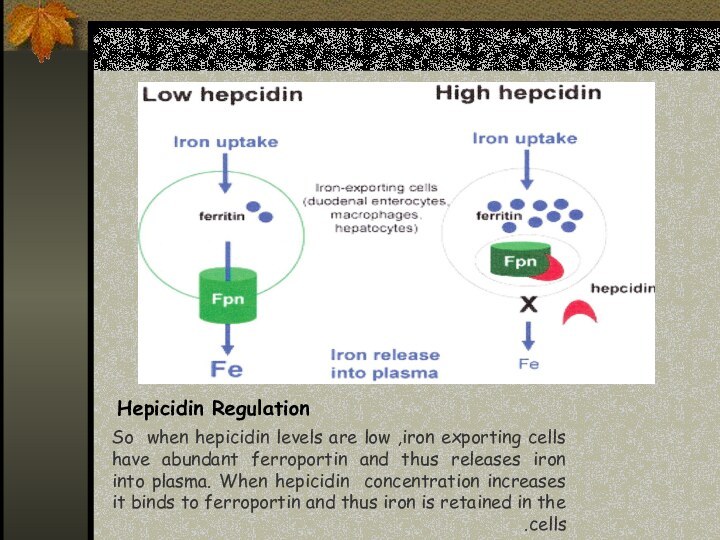
Hepicidin Regulation
So when hepicidin levels are low ,iron
exporting cells have abundant ferroportin and thus releases iron
into plasma. When hepicidin concentration increases it binds to ferroportin and thus iron is retained in the cells.
Слайд 18
IRON DEFICIENCY
In 1997 Looker et al reported that
3% of American toddlers, 2-5% of American teenage
girls are iron deficient.
More than half billion people worldwide have adverse effects as a result of iron deficiency.
Слайд 19
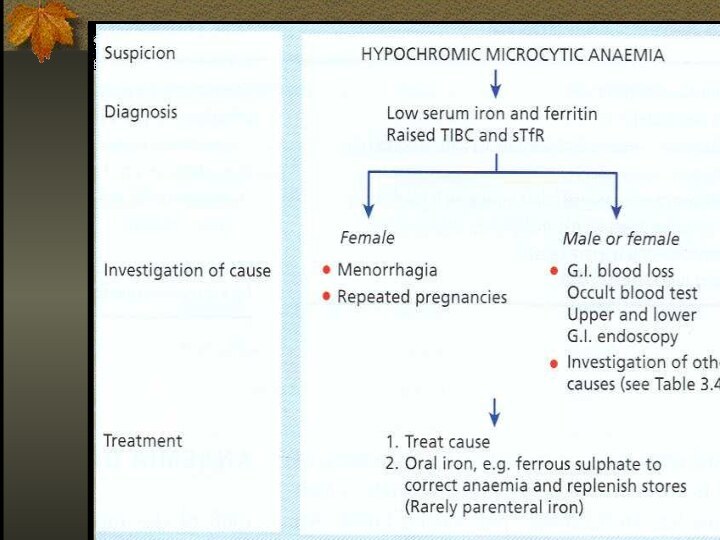
Iron deficiency is the commonest cause of anemia
world wild.
The anemia of iron deficiency is caused by
defective synthesis of hemoglobin resulting in red blood cells that are smaller than normal (microcytic), and contain reduced amounts of hemoglobin (hypo chromic).
Слайд 21
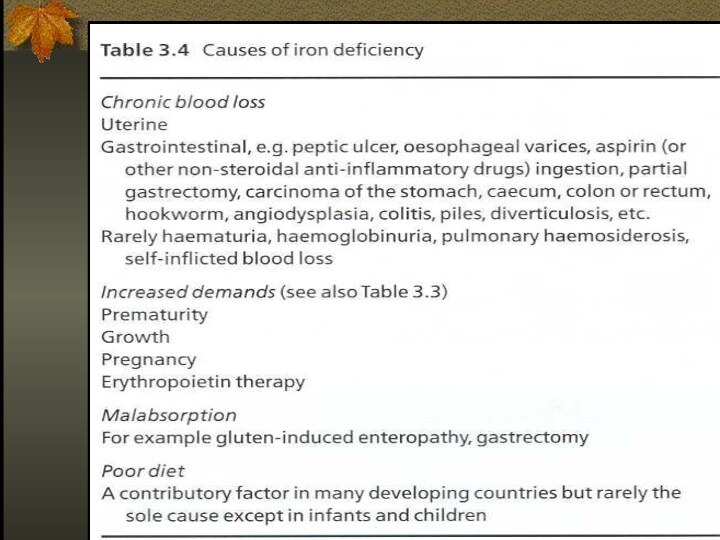
Causes of Iron Deficiency
Inadequate absorption
Antiacid or high
gastric Ph
Excess bran,phytates
Loss of enterocytes
Bowel resection
Celiac disease
Inflammatory bowel disease
Intrinsic
RBC defect
Increased loss or requirement
Growth, pregnancy, lactation
GIT loss
Genitourinary loss
Pulmonary loss
Other – trauma, excessive phlebotomy, large vascular malformation
Слайд 23
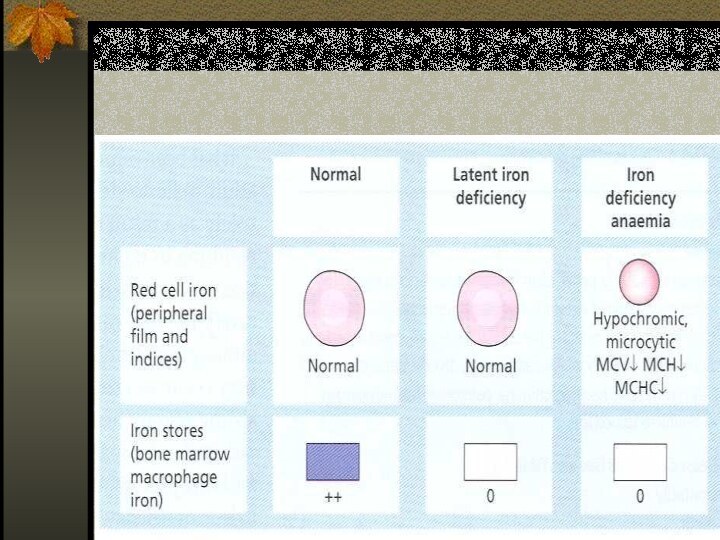
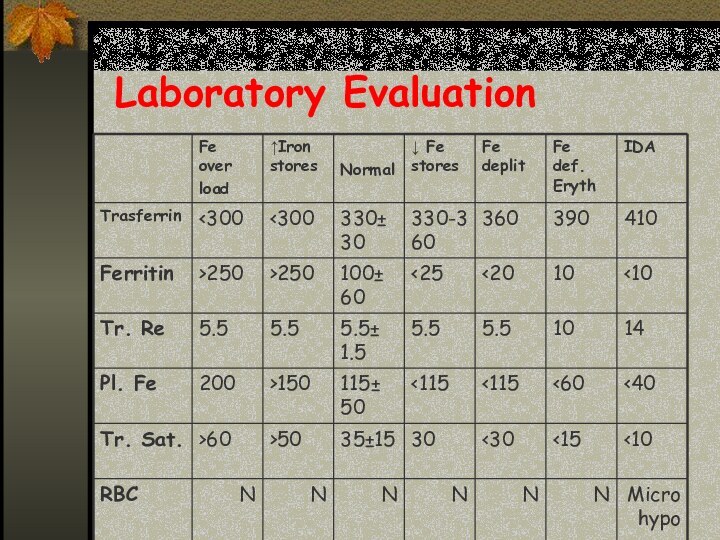
Stages of Iron Deficiency
Iron depletion - decrement of
iron stores, no decline in functional iron compound.
Iron deficient
erythropoesis – occurs when iron stores are exhausted and lack.
Frank Iron Deficiency Anemia.
Слайд 25
Clinical Presentation
Asymptomatic
Signs and symptoms of underlying disorders
Manifestations common
to anemia from all causes: pallor, weakness, shortness of
breath etc.
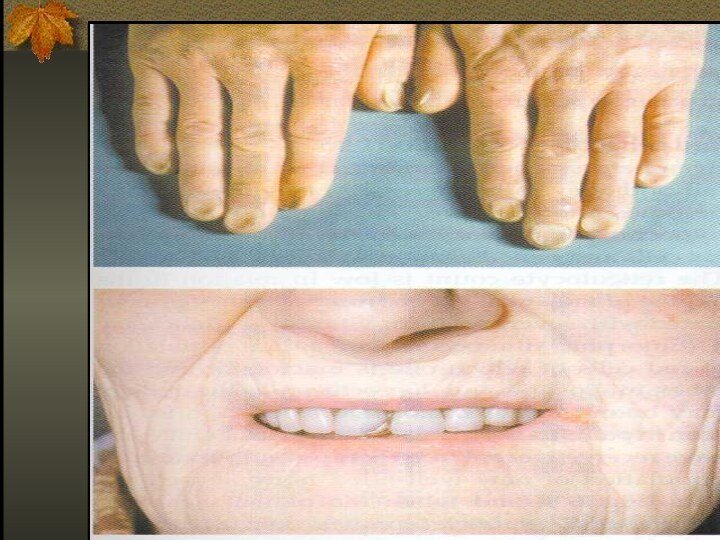
Specific to iron deficiency: cognitive abnormalities, pica, koilonychia, blue sclera, Plumer-Vinson syndrome
Слайд 29
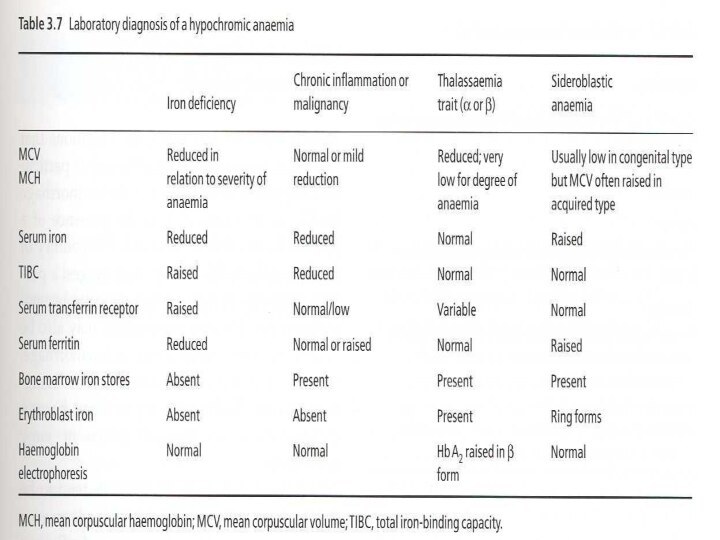
Differential Diagnosis of Microcytic Anemias
With decreased iron
stores
Iron Deficiency Anemia
With normal or increased iron
stores
Impaired iron metabolism
Anemia of chronic disease
Disorders of globin synthesis: thalassemia
Disorders of heme synthesis : sideroblastic anemia
Слайд 30
THERAPY
Therapeutic trail of iron – confirms diagnosis of
IDA if:
Reticulocytosis starts 3-5 days from therapy
Rise of Hb
10-21 days from therapy
Must make sure – compliance, stop blood loss, treat coexistent disease
Слайд 31
ORAL IRON THERAPY
Ferrous (Fe3+) iron salt supplying 150-200
mg elemental iron daily divided in 3-4 doses
In children
3mg/kg/day
Ferrous sulfate most widely used
Continue treatment for 4-6 months or until ferritin >50μg/l
Слайд 32
Parenteral Iron Therapy
Malabsorption
Intolerance to oral treatment
Chronic uncontrolled bleeding
RISKS
– anaphylaxis (0.5-1%), severe serum sickness, given IM –
local reactions and staining
DOSAGE – iron dextrane 50mg/l elemental iron, total dose calculated from iron body deficit to correct Hb, not stores
Слайд 34
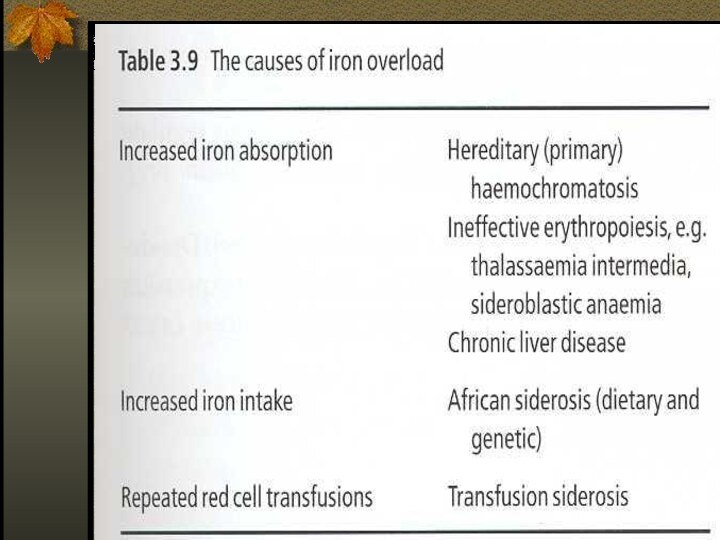
Iron Overload
Accumulation of iron can occur
in disorders associated with excessive absorption or chronic blood
transfusions
Слайд 35
Disease States
Hepcidin deficiency, physiological = Haemochromatosis
Hepcidin
excess – anaemia of chronic disease
Слайд 37
The role of Hepcidin in hereditary hemochromatosis
Hereditary hemochromatosis:
-excessive
intestinal iron absorption
-Saturation of transferrin
-Iron deposition in vital organs
Слайд 38
Hereditary Hemochromatosis
Autosomal recessive disease
Excessive absorption of Fe
from GIT
HFE – the gene involved, situated close to
MHC locus on chromosome 6 and associated with HLA-A3 and –B8
The consequence of mutation in HFE, it is not expressed on duodenal crypt cells and isn’t able to incorporate iron and seems iron deficient and absorbs more iron
Down regulation of hepcidin
Слайд 39
Iron Overload
The clinical features of iron overload from
any cause are similar:
- skin hyper pigmentation
- endocrine abnormalities: diabetes mellitus, gonadal, thyroid, pituitary and parathyroid dysfunction
- liver fibrosis, cirrhosis, hepatocellular carcinoma
- cardiomyopathy
- arthropathy
Слайд 40
Therapy
Hemochromatosis without anemia – regular venesection, each
unit of blood removes 200-250 mg of iron, with
monitoring of Fe, TIBC, Ferritin
Transfusional iron overload – with Fe chelators that cause to excretion of iron in urine or feces.
Слайд 41
Iron chelators
Deferoxamine – parenteral use, excretion in urine,
side effects – deafness, visual, growth, and bone abnormalities
Deferiprone
– oral, 3/d alone or with deferoxamine, urine exretion, more effective in cardiac iron deposition, side effects – arthropathy, agranulocytosis (1%)
Deferasirox (Exjade) – oral, fecal excretion side effects mild – skin rashes, transient liver enzymes elevation