Слайд 3
Early Concepts, Pre 1950.
Fifty years ago, biomedical scientists

were certain that there was an impervious barrier between
the immune system and the brain. The barrier, called the blood-brain barrier, was thought to protect the brain from any effects of the immune system. It was not thought possible for immune cells to migrate from the blood, through the blood-brain barrier and into the brain. According to the prevailing view of the time, immune cells did not reside in the brain either. The concept of the immune system releasing chemical messengers which traveled through the blood and into the brain had absolutely no scientific support. The notion of immune cells secreting chemical messengers of any sort was immunological heresy. As a result, a model of the immune system communicating with the brain was never proposed or discussed because it was considered biologically impossible.
Communicating in the other direction, that is, from the brain to the immune system, was considered impossible also. For one thing, neuroanatomists could not find nerves extending from the brain to cells or structures of the immune system. For another, there were no reports of the brain secreting chemical messengers which could regulate the immune system. Without chemical messengers or nerve connections, the brain could not send vital information to the immune system.
Consequently, before 1950, there were no biological concepts of a functional connection between the immune system and the brain. The biological dogma of the time was: 1). The immune system cannot communicate with the brain or control any brain function; 2). The brain cannot communicate with the immune system or control any immune system functions. In short, there was no hypothesis of an immune-brain connection before 1950.
Слайд 4
In the 1950's, biologists discovered that hormones help
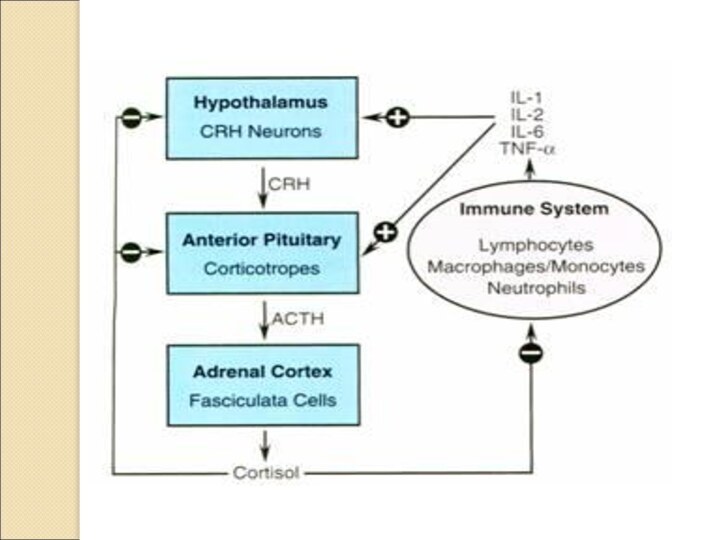
regulate the immune system. Cortisol (also called hydrocortisone) is
the best known example of hormonal control of the immune system. It is an anti-inflammatory and immunosuppressive hormone made by the adrenal cortex. The production of cortisol is governed by the pituitary and the pituitary is controlled by the hypothalamus. Therefore another revolutionary conclusion: the brain, via its control over cortisol and other hormone secretions, helps regulate the immune system.
Many hormones influence the immune system. In fact, most of them do. The hypothalamus, for example, secretes many potent hormones and a number of them help control immune cells. The pituitary secretes many different hormones and these hormones help regulate immune cells. In like manner, sex hormones secreted by the ovaries and testes influence immune cells. So do thyroid hormones.
The brain and peripheral nerves release numerous neurotransmitters and other chemicals called neuropeptides. Neurotransmitters and neuropeptides are not usually called hormones, but they do have hormone like properties, that is, they are chemical messengers. Most neurotransmitters and neuropeptides influence immune cell activities. As you can see, there are many hormones, neurotransmitters and neuropeptides released by the brain or by structures controlled by the brain which regulate the immune system.
Слайд 5
Neurotransmitters and hormones are chemicals secreted inside our brain

and are largely responsible for our behavior and attitude.
There are many similarities in the two compounds that make people think they are one and the same whereas in reality there are great differences between a neurotransmitter and a hormone that need to be appreciated.
The most notable difference between a neurotransmitter and a hormone pertains to the point of its release inside the body. A hormone is a compound produced by endocrine gland and is released directly into the bloodstream where it easily finds its target cells at a small distance from the point of release. On the other hand, a neurotransmitter is a compound released by a nerve terminal when the nerve is triggered by an electrical impulse. As this electrical impulse reaches the end of the nerve, it secretes a chemical compound at a special place in between the nerve cells called synapse. In comparison to hormones that take time to have their effect; these nerve cells are in direct apposition with the target cells which ensures quick delivery of the signal.
There are receptors for both hormones as well as neurotransmitters in the target cells and these receptors induce biochemical responses from the individual depending upon the type of hormone or neurotransmitter. Thus the difference between a neurotransmitter and a hormone boils down to the release mechanism and this mechanism alone decides whether the released molecule is a hormone or a neurotransmitter. Thus adrenaline is a hormone secreted by adrenaline gland directly into the bloodstream which goes to heart and the lungs. On the other hand serotonin is a neurotransmitter as it is released by a stimulated presynaptic nerve cell and acts on its neighboring postsynaptic cell.
Both hormones and neurotransmitters are vital for human beings as they play an important role in many bodily processes such as digestion, metabolism, reproduction etc. They are also important in mood control. Some people are more aggressive than others and it is a result of secretion of higher amounts of some hormones and neurotransmitters inside the body.
Difference between Neurotransmitters and Hormones
• Both neurotransmitters and hormones are chemicals secreted inside our bodies.
• While hormones are produced by endocrine gland, neurotransmitters are produced at nerve terminals when triggered by an electrical impulse.
• Hormones are secreted directly into the bloodstream whereas neurotransmitters are secreted at nerve synapses.
• Hormones can be synthesized whereas it is impossible to make neurotransmitters. They are made inside the body only.
Слайд 6
In addition to the extensive ability to chemically

(i.e. via hormones, neurotransmitters and neuropeptides) regulate the immune
system, the brain can also directly control important parts of the immune system through its network of nerves. Starting in the 1960's, neuroanatomical investigations began finding direct nervous links between the brain and the immune system. There are nerves going directly from the brain to important immune organs like the thymus, bone marrow, spleen, lymph nodes and gut associated lymphoid tissue. By having nerves connected to these important immune organs, the brain is able to directly regulate immune system activities. Extensive animal studies have shown that the brain, via its nerve connections, does exert significant control over these immune organs.
Thus, from 1950 to 1978, a radically changed view of the immune-brain connection developed. Massive hormonal and neuroanatomical evidence made it clear that there was a connection between the brain and the immune system. The brain, through its direct nerve connections to the immune system and its control over the extensive hormone network, helped govern the immune system. A new biomedical discipline, called psychoneuroimmunology, grew up around these discoveries.
In 1978, the paradigm for the immune-brain connection was: 1). The brain, in a very complex way, regulated the immune system. The direction of the control was from the brain to the immune system, that is, Brain→Immune System. 2). There was no evidence the immune system could control the brain, therefore the immune-brain connection was a one-way street, Brain→Immune System, and not a two-way street, Brain↔Immune System.
Слайд 7
Before the remarkable discoveries on cytokines, it was
assumed to be impossible for the immune system to
communicate with the brain. Like no other previous discovery, cytokines have revolutionized our understanding of the communications link between the immune system and the brain. The one direction pathway model is now untenable. It is simply wrong. Instead, we now know the communications pathway is bi-directional, that is, it is a two-way street: Immune system↔Brain. There is a continuous information loop going from the immune system to the brain and from the brain back to the immune system. Thus, the immune system can control the brain and the brain can control the immune system.
Слайд 8
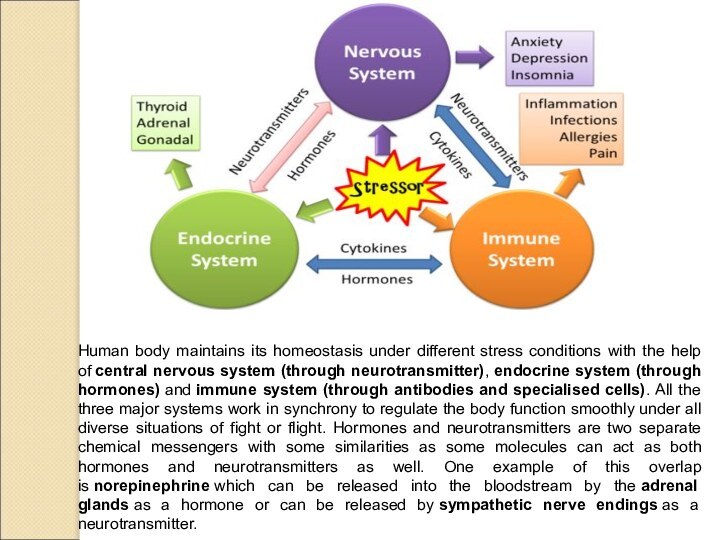
Human body maintains its homeostasis under different stress
conditions with the help of central nervous system (through neurotransmitter), endocrine
system (through hormones) and immune system (through antibodies and specialised cells). All the three major systems work in synchrony to regulate the body function smoothly under all diverse situations of fight or flight. Hormones and neurotransmitters are two separate chemical messengers with some similarities as some molecules can act as both hormones and neurotransmitters as well. One example of this overlap is norepinephrine which can be released into the bloodstream by the adrenal glands as a hormone or can be released by sympathetic nerve endings as a neurotransmitter.
Слайд 10
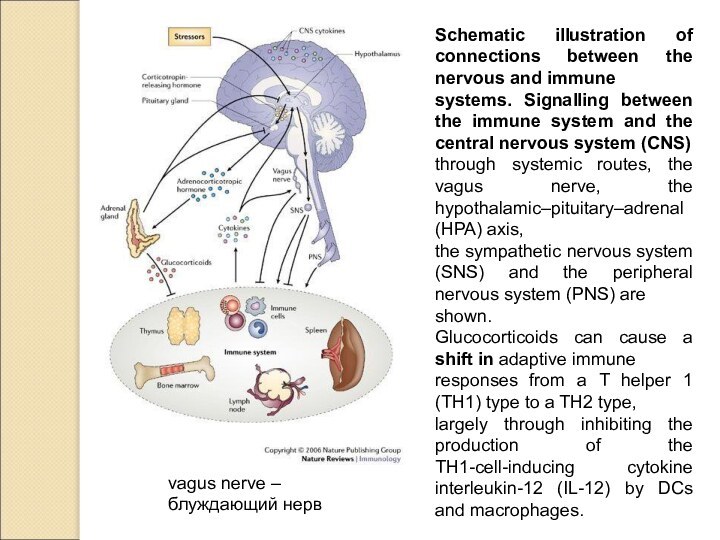
vagus nerve –
блуждающий нерв
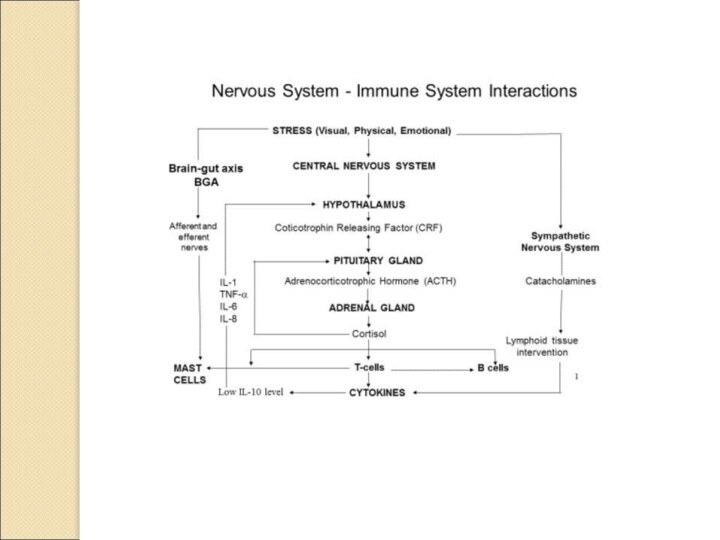
Schematic illustration of connections between
the nervous and immune
systems. Signalling between the immune system
and the central nervous system (CNS)
through systemic routes, the vagus nerve, the hypothalamic–pituitary–adrenal (HPA) axis,
the sympathetic nervous system (SNS) and the peripheral nervous system (PNS) are
shown.
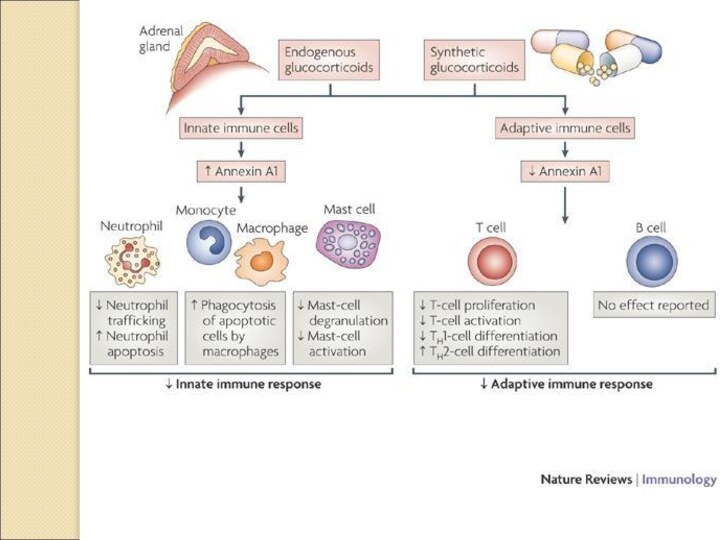
Glucocorticoids can cause a shift in adaptive immune
responses from a T helper 1 (TH1) type to a TH2 type,
largely through inhibiting the production of the TH1-cell-inducing cytokine interleukin-12 (IL-12) by DCs and macrophages.
Слайд 11
Glucocorticoid effects on innate immune-cell function.
Glucocorticoids suppress maturation,
differentiation and proliferation of all immune cells, including Dendritic
Cells (DC) and macrophages. Glucocorticoids inhibit DC differentiation depending on the stage of
maturation and the DC subtype.
Glucocorticoids act on immune cells both directly and indirectly to suppress the induction of proinflammatory responses. They inhibit the production of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumour-necrosis factor (TNF), while promoting the production of anti-inflammatory cytokines, such as IL-10, by macrophages and dendritic
cells. They also promote apoptosis of macrophages, dendritic cells and T cells, leading to inhibition of immune responses. IFNγ, interferon-γ; NK cell, natural killer cell; TC, cytotoxic T cell; TH, T helper cell.
Слайд 15
Cytokines and the Immune-Brain Connection After the scientific acceptance

of cytokines in 1979 and the availability of pure
recombinant cytokines, it became apparent over the next few years that the immune system can send powerful chemical messages to the brain. Receptors for IL-1, IL-2, IL-6 and a few other cytokines were discovered throughout the brain. These cytokines were found to be able to travel in the blood, through the blood-brain barrier and into the brain. When animals or humans were given various cytokines intravenously, dramatic changes in behavior and brain function occurred. The above observations demonstrate the ability of cytokines to pass through the blood brain barrier and profoundly influence brain function.
Recent investigations have revealed peripheral nerves as another pathway for cytokines to deliver messages to the brain. Many peripheral nerves have receptors for cytokines. In animal experiments, IL-1 activates peripheral nerves, thereby forcing the nerves to send messages directly to the brain.
These cytokine experiments indicate that activated immune cells in the skin, stomach, throat or any other site, can send urgent, powerful messages to the brain by secreting cytokines into the blood or into tissue spaces near certain peripheral nerves. This explains why an infection or other pathology in the throat, bladder, liver, stomach (ulcers, for example) or any site can profoundly affect brain function and behavior. Any pathology that activates the immune system can affect brain function and behavior.
Слайд 16
The Immune System as a Sensory Organ.
The two-way
communications model has permitted immunologists to look at the
immune system in completely new ways. One very novel way is to view the immune system as a sensory organ. This shouldn't be surprising, since immune cells are constantly on alert to detect dangerous bacteria, viruses, fungi, foreign proteins, antigens, harmful chemicals, poisons, malignant cells, damaged tissue, dying cells and abnormal cells. In other words, the immune system is constantly 'sensing' for danger at the chemical and cellular level.
The immune system's sensory function goes on 24 hours a day. In every tissue, including throat, lung, liver, stomach, brain, skin, kidney and blood, immune cells are constantly on alert for danger. When immune cells sense danger, they become activated and start secreting various cytokines to inform neighboring cells about the danger. Nearby peripheral nerves, if they have cytokine receptors, will carry the cytokine message to the brain. In addition, if enough cytokine is secreted to spill into the blood, then every tissue and organ in the body, including the brain, will be directly informed of the danger.
Слайд 17
The Immune-Brain Connection & The Six Senses.
The two-way
model (Immune System↔Brain) shows that both systems can communicate
with each other, but it doesn't indicate which system initiates the communication. Most likely the immune system sends the first message since the immune system is a sensory organ and it is the function of sensory organs to send new information the brain. Then the brain would respond to the new sensory information by sending messages back to the immune system. The messages from the brain would help the immune system coordinate its defense of the body. This crosstalk cycle could be repeated over and over again. Each cycle would be purposeful, with the immune system sending new, urgent information to the brain on chemical-microbiological dangers and the brain responding with information to help coordinate the defenses against the dangers. In diagram form the information flow would be:
6th Sense (Immune System) → Brain ↔ Immune System
Слайд 21
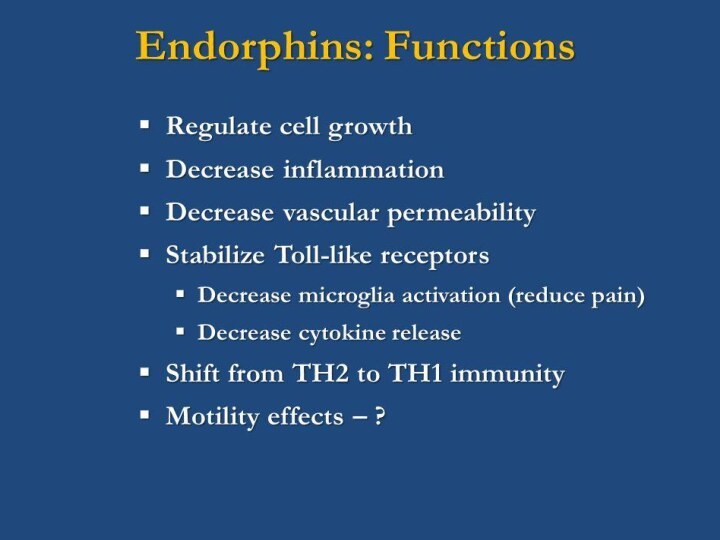
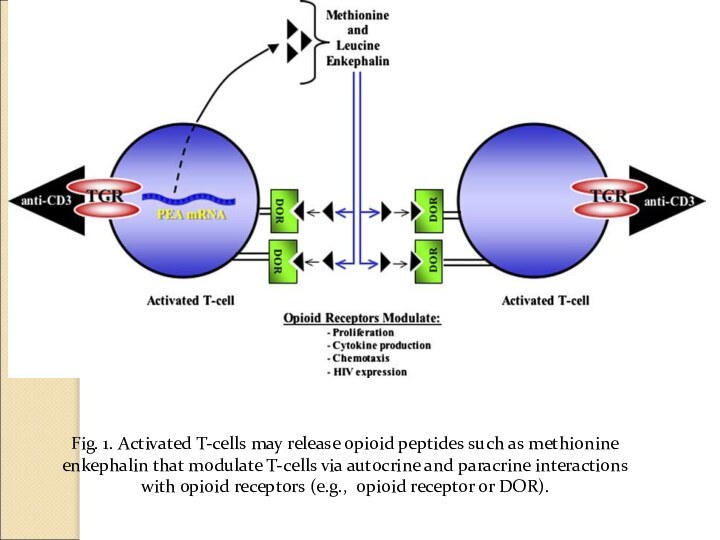
Fig. 1. Activated T-cells may release opioid peptides
such as methionine enkephalin that modulate T-cells via autocrine
and paracrine interactions
with opioid receptors (e.g., opioid receptor or DOR).
Слайд 22
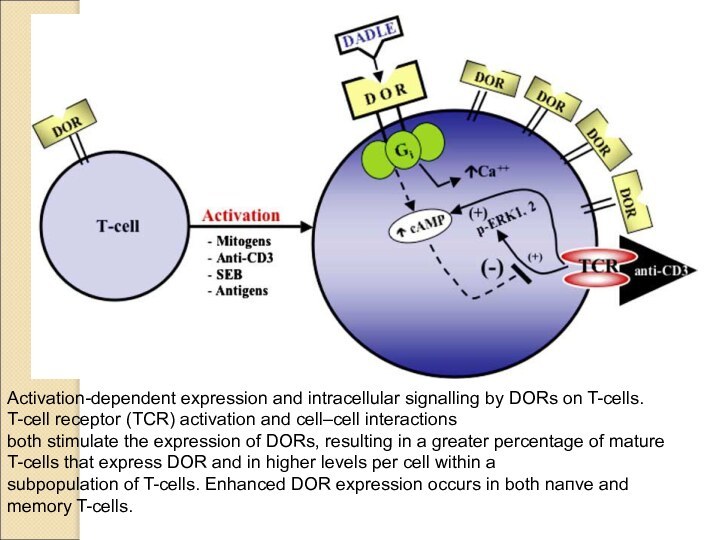
Activation-dependent expression and intracellular signalling by DORs on
T-cells. T-cell receptor (TCR) activation and cell–cell interactions
both stimulate
the expression of DORs, resulting in a greater percentage of mature T-cells that express DOR and in higher levels per cell within a
subpopulation of T-cells. Enhanced DOR expression occurs in both naпve and memory T-cells.