- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Биофармацевтика, занятие 6Факторы роста – ЭФР, тромбоцитарный фактор роста, ФРФ, ТРФ, нейротропные ростовые факторы
Содержание
- 2. Заживление ранStuart Enoch, Patricia Price. Cellular, molecular
- 3. Заживление ранHaemostasis he alpha granules of the
- 4. Заживление ран – ростовые факторыCytokines in Wound Healing in R&D Systems' 2002 catalog
- 5. Singer AJ, Clark RA. Cutaneous wound healing.
- 6. Figure 2. A cutaneous wound five days
- 7. Синдром диабетической стопыStuart Enoch, Patricia Price. Cellular,
- 8. Эпидермальный фактор роста - ЭФРФактор роста фибробластов
- 9. Эпидермальный фактор ростаЭФР, 6 кДа, 53 а.к.,
- 10. Рецептор ЭФР
- 11. Путь передачи сигнала ЭФРКомплекс с рецептором 2:2MAPK/ERK
- 12. Путь передачи сигнала ЭФР© Jorge Berlanga-Acosta, Jorge
- 13. Лекарственная форма ЭФРТорговое название REGEN-D, мазь, разрешена
- 14. Экспрессия рецептора ЭФР клетками опухолейTable 1: Frequency of
- 15. Тромбоцитарный фактор роста5 гомологов, 4 гомодимера PDGF-A,
- 16. Рецептор ТцФР
- 17. ТцФР - путь передачи сигнала
- 18. ТцФР при заживлении ранFigure 2: PDGF signal amplification
- 19. Лекарственная форма ТцФР Торговое название Регнарекс гель,
- 20. Клиническая эффективность ТцФРFig. 2. Estimated probability of complete
- 21. Канцерогенез, вызываемый ТцФРAdv Skin Wound Care. 2011 Jan;24(1):31-9.
- 22. Семейство факторов роста фибробластовОколо 20 членов, ФРФ-1
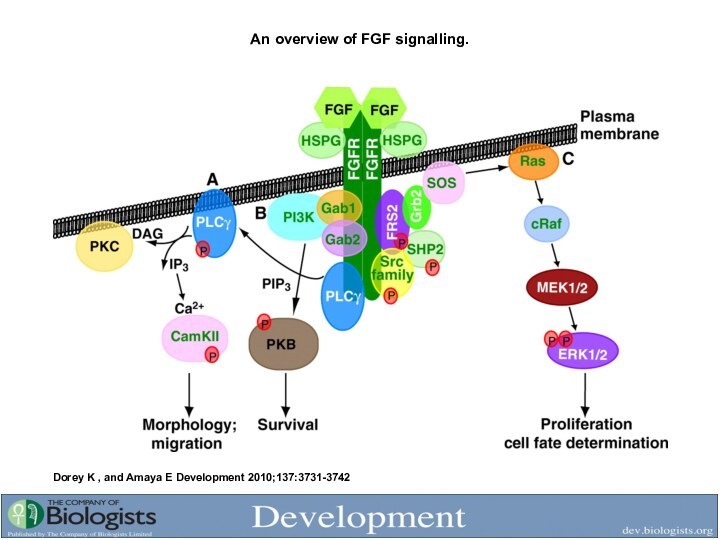
- 23. An overview of FGF signalling. Dorey K , and Amaya E Development 2010;137:3731-3742
- 24. Палифермин (ФГФ-7) и его рецептор.Фактор роста кератиноцитов
- 25. Лекарственный препарат ФГФ-7Торговое название Kepivance, МНН PaliferminПоказания
- 26. Трансформирующие ростовые факторыДва члена семейства – TGF-α
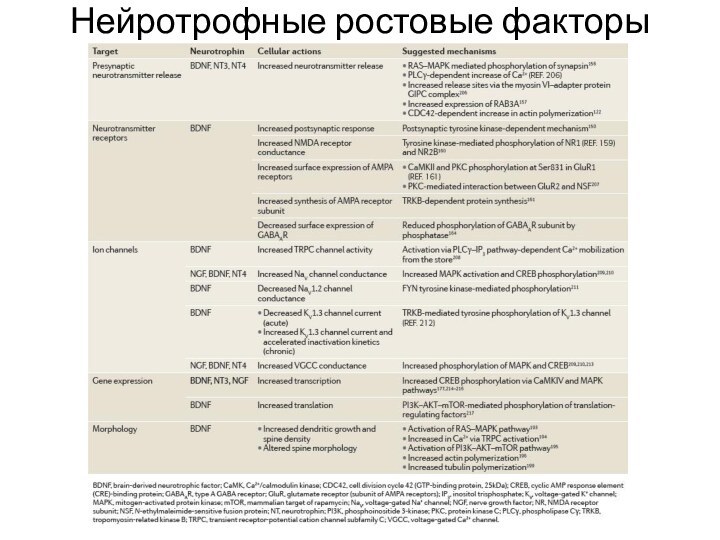
- 27. Нейротрофные ростовые факторы
- 28. Рецептор NGFa | On binding of nerve growth
- 29. Роль нейротрофных ростовых факторов при нейродегенеративных заболеванияхPLoS
- 30. Скачать презентацию
- 31. Похожие презентации
Заживление ранStuart Enoch, Patricia Price. Cellular, molecular and biochemical differences in the pathophysiology of healing between acute wounds, chronic wounds and wounds in the aged // World Wide Wounds, online Aug 2004

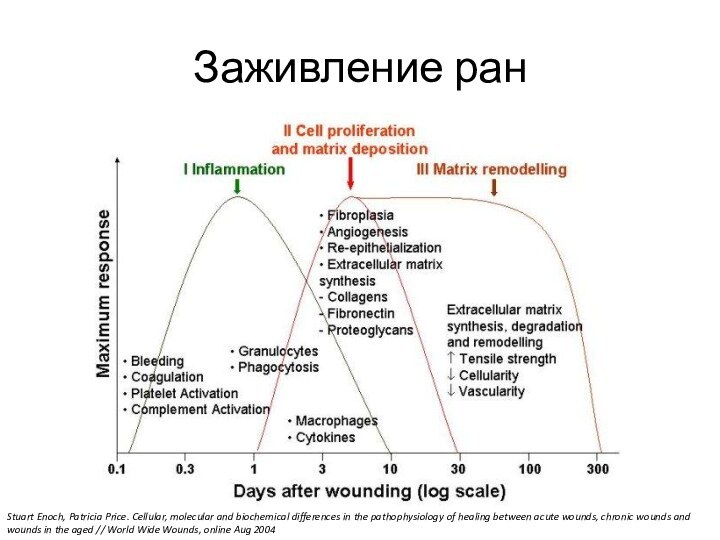












Слайд 3
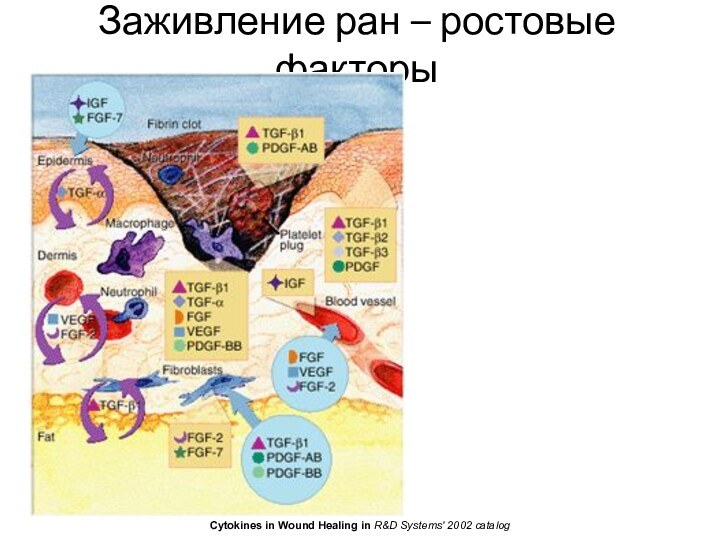
Заживление ран
Haemostasis he alpha granules of the platelets
- PDGF, IGF-1, EGF, TGF-β. These proteins initiate the
wound healing cascade by attracting and activating fibroblasts, endothelial cells and macrophages.Early inflammatory phase activation of complement, infiltration of the wound with granulocytes or polymorphonuclear leucocytes (PMNLs). attracted by C5a, platelets, TGF-β. cleared away by extrusion to the wound surface
Late inflammatory phase Blood monocytes to tissue macrophages. Monocytes attracted by complement, clotting components, IgG fragments, collagen and elastin breakdown products, leukotriene B4, platelet factor IV, PDGF and TGF-β. release further cytokines and growth factors into the wound, recruiting fibroblasts, keratinocytes and endothelial cells. release collagenase, secrete TGF-α, heparin-binding epidermal growth factor (HB-EGF) bFGF. Lymphocyte (>72 h) attracted by IL-1, IgG and complement. IL-1 regulates collagenase, thus remodeling extracellular matrix (ECM).
Proliferative phase Granulation tissue.
Fibroblast migration: Fb produce the matrix proteins fibronectin, hyaluronan (HA) and later collagen and proteoglycans. Attracted by PDGF and TGF-β, construct the new ECM.
Collagen synthesis: By Fb - types I and III collagen to form the new matrix.
Angiogenesis: By releasing of TGF-β and PDGF by platelets, attract macrophages and granulocytes. Mp releases tumour necrosis factor-α; and bFGF.
Granulation tissue formation:
Epithelialisation: EGF for mitogenesis and chemotaxis, bFGF and KGF- epithelial proliferation.
Remodelling phase Collagen degradation by MMPs, produced by Fb, granulocytes, Mp. Cut by TIMPs. Mediated by TGF-β. Contraction by Fb and ECM
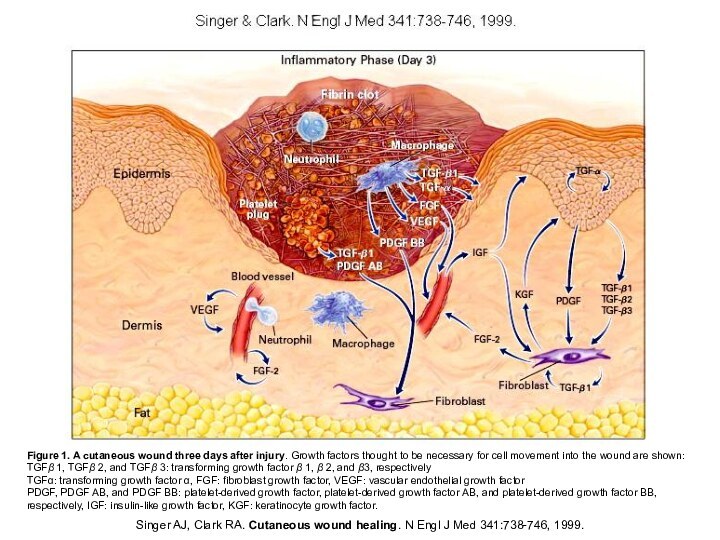
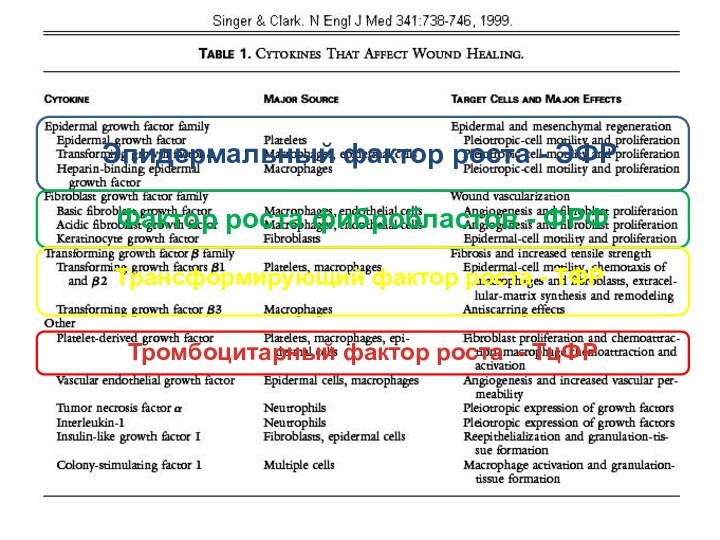
Слайд 5 Singer AJ, Clark RA. Cutaneous wound healing. N
Engl J Med 341:738-746, 1999.
Figure 1. A cutaneous wound
three days after injury. Growth factors thought to be necessary for cell movement into the wound are shown:TGFβ 1, TGFβ 2, and TGFβ 3: transforming growth factor β 1, β 2, and β3, respectively
TGF: transforming growth factor , FGF: fibroblast growth factor, VEGF: vascular endothelial growth factor
PDGF, PDGF AB, and PDGF BB: platelet-derived growth factor, platelet-derived growth factor AB, and platelet-derived growth factor BB, respectively, IGF: insulin-like growth factor, KGF: keratinocyte growth factor.
Слайд 6 Figure 2. A cutaneous wound five days after
injury. Blood vessels are seen sprouting into the fibrin
clot as epidermal cells resurface the wound. Proteinases thought to be necessary for cell movement are shown.u-PA: urokinase-type plasminogen activator; MMP-1, 2, 3, and 13: matrix metalloproteinases 1, 2, 3, and 13 (collagenase 1, gelatinase A, stromelysin 1, and collagenase 3, respectively); t-PA: tissue plasminogen activator.
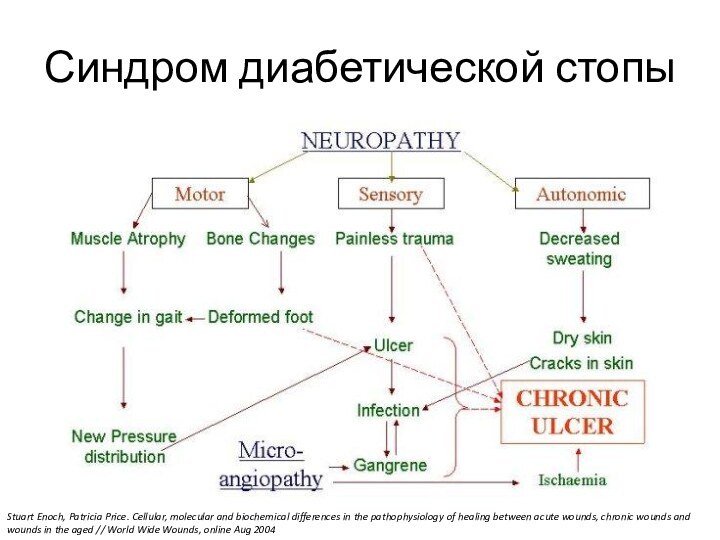
Слайд 7
Синдром диабетической стопы
Stuart Enoch, Patricia Price. Cellular, molecular
and biochemical differences in the pathophysiology of healing between
acute wounds, chronic wounds and wounds in the aged // World Wide Wounds, online Aug 2004
Слайд 8
Эпидермальный фактор роста - ЭФР
Фактор роста фибробластов -
ФРФ
Трансформирующий фактор роста - ТФР
Тромбоцитарный фактор роста - ТцФР
Слайд 9
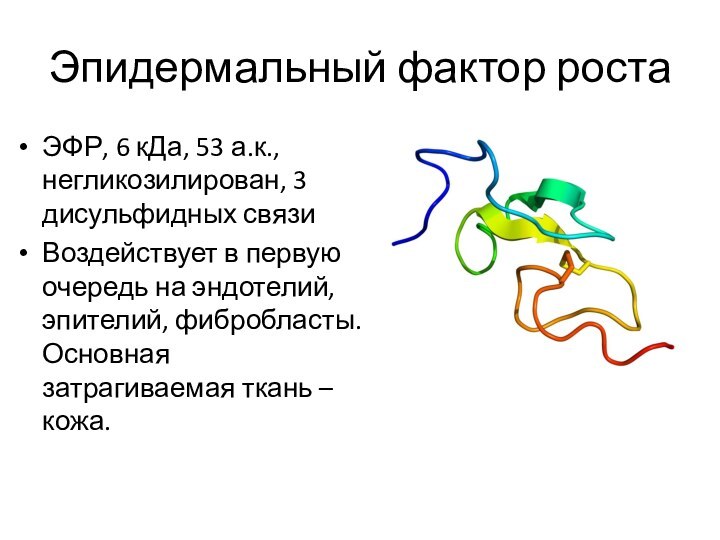
Эпидермальный фактор роста
ЭФР, 6 кДа, 53 а.к., негликозилирован,
3 дисульфидных связи
Воздействует в первую очередь на эндотелий, эпителий,
фибробласты. Основная затрагиваемая ткань – кожа.
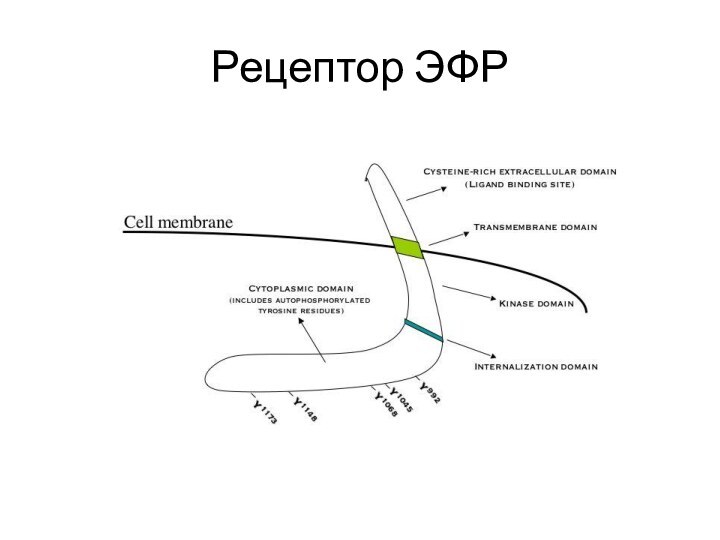
Слайд 11
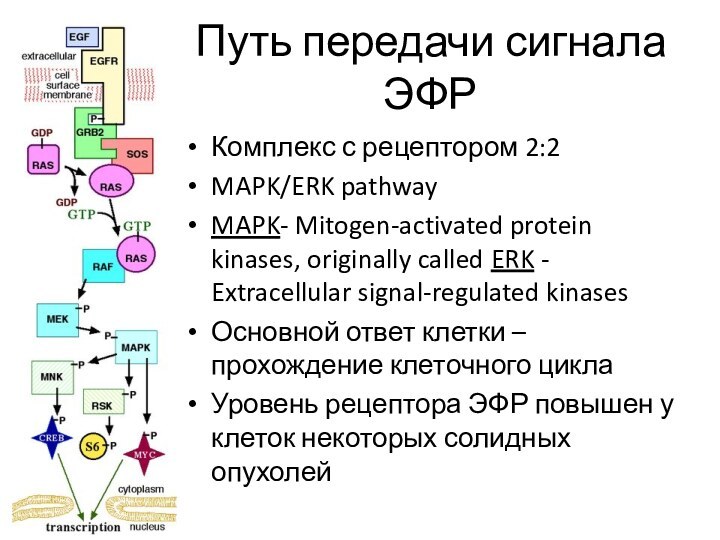
Путь передачи сигнала ЭФР
Комплекс с рецептором 2:2
MAPK/ERK pathway
MAPK-
Mitogen-activated protein kinases, originally called ERK - Extracellular signal-regulated
kinasesОсновной ответ клетки – прохождение клеточного цикла
Уровень рецептора ЭФР повышен у клеток некоторых солидных опухолей
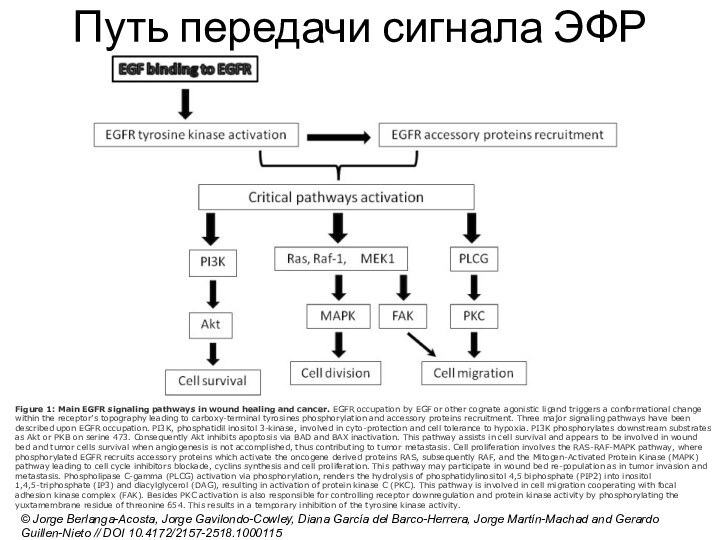
Слайд 12
Путь передачи сигнала ЭФР
© Jorge Berlanga-Acosta, Jorge Gavilondo-Cowley,
Diana García del Barco-Herrera, Jorge Martín-Machad and Gerardo Guillen-Nieto
// DOI 10.4172/2157-2518.1000115
Слайд 13
Лекарственная форма ЭФР
Торговое название REGEN-D, мазь, разрешена для
клинического применения только в Индии, 150 мкг/г ЭФР.
Показания –
язва при синдроме диабетической стопыЭффективность определена как уменьшение времени заживления язвы против плацебо
Нет данных о наблюдениях за канцерогенезом
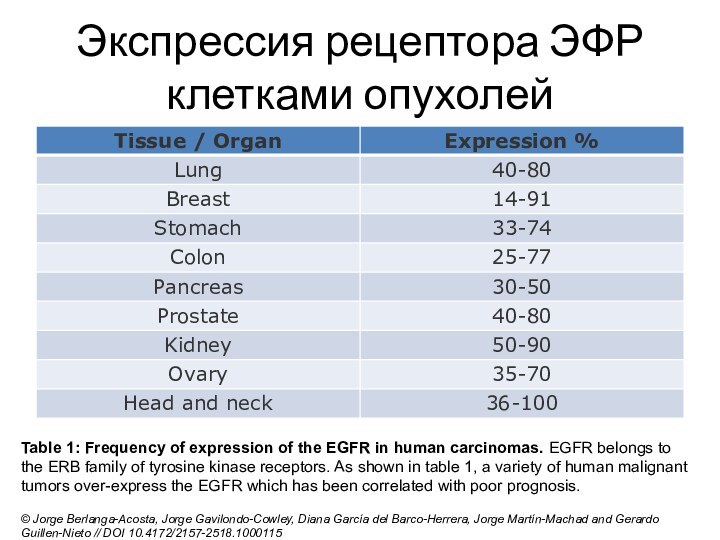
Слайд 14
Экспрессия рецептора ЭФР клетками опухолей
Table 1: Frequency of expression
of the EGFR in human carcinomas. EGFR belongs to the
ERB family of tyrosine kinase receptors. As shown in table 1, a variety of human malignant tumors over-express the EGFR which has been correlated with poor prognosis.© Jorge Berlanga-Acosta, Jorge Gavilondo-Cowley, Diana García del Barco-Herrera, Jorge Martín-Machad and Gerardo Guillen-Nieto // DOI 10.4172/2157-2518.1000115
Слайд 15
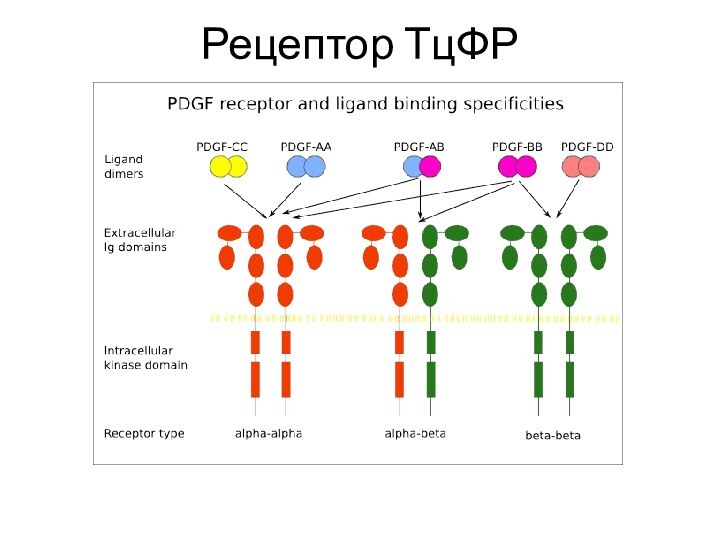
Тромбоцитарный фактор роста
5 гомологов, 4 гомодимера PDGF-A, -B
… -D, гетеродимер PDGF-A-B
Для PDGF-A 1 сайт гликозилирования, 3
дисульфидных связи. 110 а.к., 125 а.к.Синтезируются мегакариоцитами, хранятся в альфа-гранулах тромбоцитов
Основные мишени – фибробласты, гладкая мускулатура, глиальные клетки
Основное действие - паракринное
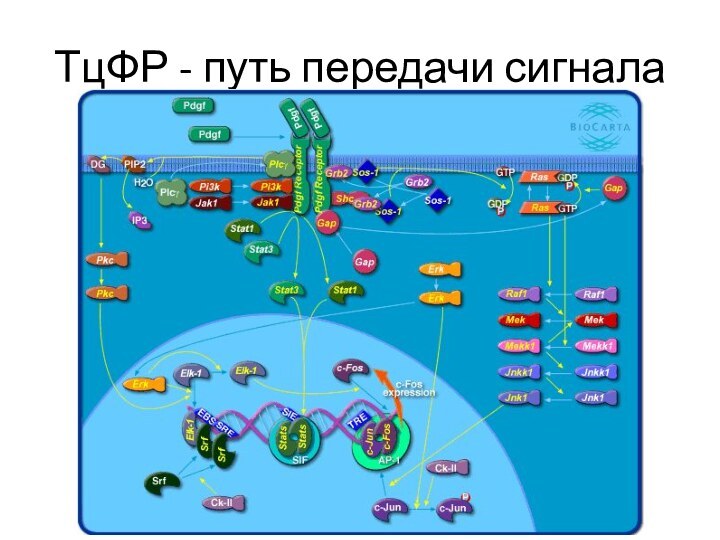
Слайд 18
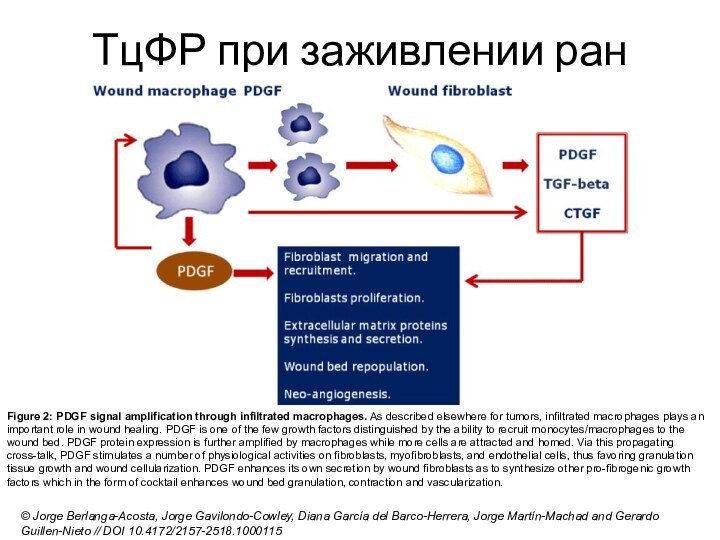
ТцФР при заживлении ран
Figure 2: PDGF signal amplification through
infiltrated macrophages. As described elsewhere for tumors, infiltrated macrophages plays
an important role in wound healing. PDGF is one of the few growth factors distinguished by the ability to recruit monocytes/macrophages to the wound bed. PDGF protein expression is further amplified by macrophages while more cells are attracted and homed. Via this propagating cross-talk, PDGF stimulates a number of physiological activities on fibroblasts, myofibroblasts, and endothelial cells, thus favoring granulation tissue growth and wound cellularization. PDGF enhances its own secretion by wound fibroblasts as to synthesize other pro-fibrogenic growth factors which in the form of cocktail enhances wound bed granulation, contraction and vascularization.© Jorge Berlanga-Acosta, Jorge Gavilondo-Cowley, Diana García del Barco-Herrera, Jorge Martín-Machad and Gerardo Guillen-Nieto // DOI 10.4172/2157-2518.1000115
Слайд 19
Лекарственная форма ТцФР
Торговое название Регнарекс гель, МНН
Becaplermin
Гель, действующее вещество – гомодимер ТцФР-B
Продуцент – дрожжи S.cerevisae,
гликозилированный продукт секретируется в ростовую среду, мономеры связаны двумя дисульфидными связямиНестерильные тубы по 15 г геля, 100 мкг/г субстанции, носитель – 0,01% карбоксиметилцеллюлозы, вспомогательные вещества – NaCl, NaOAc, HOAc, метилпарабен, пропилпарабен, м-крезол, L-лизин
Стабильность белка в лекарственной форме обеспечивается его иммобилизацией на отрицательно заряженных нитях КМЦ, восстанавливающие группы на конце полимера КМЦ нейтрализованы свободными аминокислотами – лизином. (патент США 5,457,093)
Оказывает воздействие только на толщину грануляционной ткани, показания – язвы при диабете, курс лечения ~20 недель.
Слайд 20
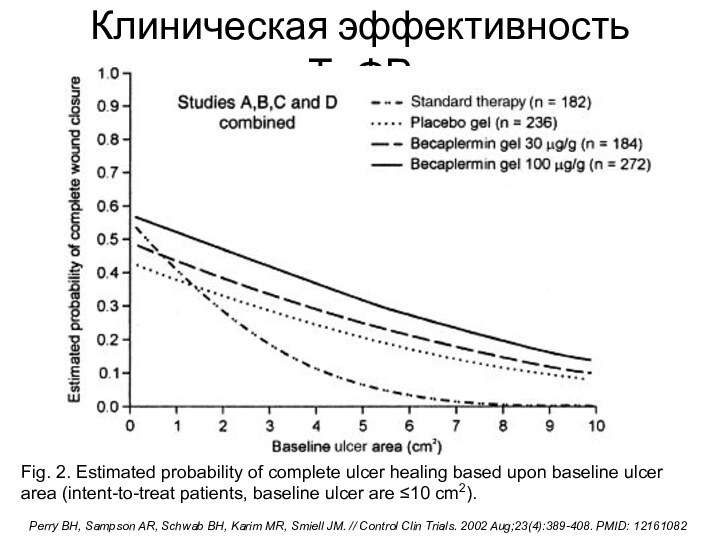
Клиническая эффективность ТцФР
Fig. 2. Estimated probability of complete ulcer
healing based upon baseline ulcer area (intent-to-treat patients, baseline
ulcer are ≤10 cm2).Perry BH, Sampson AR, Schwab BH, Karim MR, Smiell JM. // Control Clin Trials. 2002 Aug;23(4):389-408. PMID: 12161082
Слайд 21
Канцерогенез, вызываемый ТцФР
Adv Skin Wound Care. 2011 Jan;24(1):31-9. doi:
10.1097/01.ASW.0000392922.30229.b3.
A matched cohort study of the risk of cancer in users
of becaplermin.Ziyadeh N, Fife D, Walker AM, Wilkinson GS, Seeger JD.
Abstract
BACKGROUND:
Becaplermin is recombinant human platelet-derived growth factor for topical administration that might plausibly be related to cancer risk. Extended follow-up of patients from clinical trials of becaplermin compared with placebo identified a relative risk of cancer of 2.8 (95% confidence interval [CI], 0.6-12.8). The authors aimed to further investigate any association between becaplermin use and the occurrence of cancer by following a large cohort of patients in a clinical practice setting.
METHODS:
In a cohort of insured people, becaplermin initiators were matched to similar people who did not initiatebecaplermin and were followed for up to 6 years for cancer incidence (up to 9 years for cancer mortality). Cancerincidence was identified from health insurance claims and validated by review of medical records. Cancer mortality was identified through linkage to the National Death Index.
RESULTS:
Among 1622 becaplermin initiators, there were 28 confirmed cancers and 9 cancer deaths, and among the 2809 matched comparators, there were 43 confirmed cancers and 16 cancer deaths. There was no increased risk of cancer with becaplermin (hazard ratio, 1.2; 95% CI, 0.7-1.9). Cancer mortality through 2003 was increased (rate ratio [RR] = 5.2; 95% CI, 1.7-17.6) among subjects with 3 or more dispensings. Additional follow-up through 2006 indicated no elevated cancer mortality risk overall (RR, 1.0; 95% CI, 0.5-2.3) and no statistically significant increase in the subgroup with more than 3 dispensings (RR, 2.4; 95% CI, 0.8-7.4).
CONCLUSIONS:
Becaplermin does not appear to increase the risk of cancer or cancer mortality.
Слайд 22
Семейство факторов роста фибробластов
Около 20 членов, ФРФ-1 …
ФРФ-20, 18-28 кДа
Обладают общим доменом размером около 140 а.к.
Плотно
связывают гепарин и гепарин-подобные глюкозоаминогликаны, т.е. связаны с внеклеточным матриксомНекоторые члены семейства не воздействуют на фибробласты
Слайд 24
Палифермин (ФГФ-7) и его рецептор.
Фактор роста кератиноцитов (KGF,
FGF-7) воздействует только на эпителиальные клетки, обладающие рецептором FGFRIIIb
Природный
ФГФ-7 содержит 163 а.к. и сайт N-гликозилирования в N-концевой областиДелеция 23 а.к. на N-конце зрелого полипептида ФГФ-7 приводит к потере сайта N-гликозилирования и двукратному увеличению митогенной активности
Слайд 25
Лекарственный препарат ФГФ-7
Торговое название Kepivance, МНН Palifermin
Показания –
риск развития тяжелого орального мукозита вследствие высокодозной химиотерапии или
лучевой терапии с последующей пересадкой гематопоэтических стволовых клетокКлиническая эффективность – 20% больных с мукозитом уровня 4 против 62% в группе плацебо.
Курс – 6 дней по одной инъекции в/в 60 мкг/кг/д
Метод получения – E.coli, растворимый продукт в цитоплазме, окисление дисульфидной связи в процессе очистки
Слайд 26
Трансформирующие ростовые факторы
Два члена семейства – TGF-α и
три варианта TGF-β
TGF-α связан с мембраной, частично отделяется от
мембраны при протеолизе, связывается с рецептором ЭФР.TGF-β – гомодимеры, 2x112 а.к., синтезируются большинством типов клеток, плейотропны, ингибируют деление эпителиальных и гемопоэтических клеток
TGF-β стимулируют рост клеток соединительной ткани, костей и хрящей, индуцируют секрецию внеклеточного матрикса и модулируют экспрессию матриксных металлопротеаз (MMP)
Слайд 28
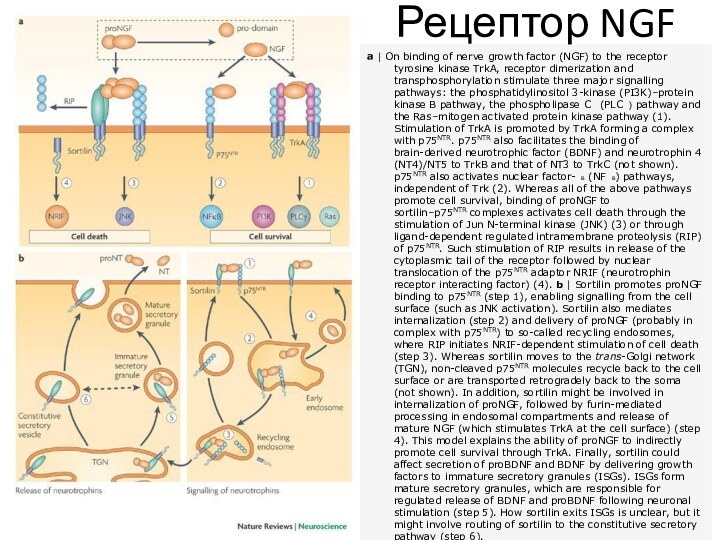
Рецептор NGF
a | On binding of nerve growth factor
(NGF) to the receptor tyrosine kinase TrkA, receptor dimerization
and transphosphorylation stimulate three major signalling pathways: the phosphatidylinositol 3-kinase (PI3K)–protein kinase B pathway, the phospholipase C (PLC ) pathway and the Ras–mitogen activated protein kinase pathway (1). Stimulation of TrkA is promoted by TrkA forming a complex with p75NTR. p75NTR also facilitates the binding of brain-derived neurotrophic factor (BDNF) and neurotrophin 4 (NT4)/NT5 to TrkB and that of NT3 to TrkC (not shown). p75NTR also activates nuclear factor- B (NF B) pathways, independent of Trk (2). Whereas all of the above pathways promote cell survival, binding of proNGF to sortilin–p75NTR complexes activates cell death through the stimulation of Jun N-terminal kinase (JNK) (3) or through ligand-dependent regulated intramembrane proteolysis (RIP) of p75NTR. Such stimulation of RIP results in release of the cytoplasmic tail of the receptor followed by nuclear translocation of the p75NTR adaptor NRIF (neurotrophin receptor interacting factor) (4). b | Sortilin promotes proNGF binding to p75NTR (step 1), enabling signalling from the cell surface (such as JNK activation). Sortilin also mediates internalization (step 2) and delivery of proNGF (probably in complex with p75NTR) to so-called recycling endosomes, where RIP initiates NRIF-dependent stimulation of cell death (step 3). Whereas sortilin moves to the trans-Golgi network (TGN), non-cleaved p75NTR molecules recycle back to the cell surface or are transported retrogradely back to the soma (not shown). In addition, sortilin might be involved in internalization of proNGF, followed by furin-mediated processing in endosomal compartments and release of mature NGF (which stimulates TrkA at the cell surface) (step 4). This model explains the ability of proNGF to indirectly promote cell survival through TrkA. Finally, sortilin could affect secretion of proBDNF and BDNF by delivering growth factors to immature secretory granules (ISGs). ISGs form mature secretory granules, which are responsible for regulated release of BDNF and proBDNF following neuronal stimulation (step 5). How sortilin exits ISGs is unclear, but it might involve routing of sortilin to the constitutive secretory pathway (step 6).
Слайд 29
Роль нейротрофных ростовых факторов при нейродегенеративных заболеваниях
PLoS One. 2010
Dec 28;5(12):e14433. doi: 10.1371/journal.pone.0014433.
Pharmacological treatment of painful HIV-associated sensory
neuropathy: a systematic review and meta-analysis of randomised controlled trials.Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS.
BACKGROUND:
Significant pain from HIV-associated sensory neuropathy (HIV-SN) affects ∼40% of HIV infected individuals treated … There is an urgent need to develop effective pain management strategies for this condition.
METHOD AND FINDINGS:
…
REVIEW METHODS:
Four authors assessed the eligibility of articles for inclusion. Agreement of inclusion was reached by consensus and arbitration. Two authors conducted data extraction and analysis. Dichotomous outcome measures (≥ 30% and ≥ 50% pain reduction) were sought from RCTs reporting interventions with statistically significant efficacies greater than placebo. These data were used to calculate RR and NNT values.
RESULTS:
Of 44 studies identified, 19 were RCTs. Of these, 14 fulfilled the inclusion criteria. Interventions demonstrating greater efficacy than placebo were smoked cannabis NNT 3.38 95%CI(1.38 to 4.10), topical capsaicin 8%, and recombinant human nerve growth factor (rhNGF). No superiority over placebo was reported in RCTs that examined amitriptyline (100mg/day), gabapentin (2.4 g/day), pregabalin (1200 mg/day), prosaptide (16 mg/day), peptide-T (6 mg/day), acetyl-L-carnitine (1g/day), mexilitine (600 mg/day), lamotrigine (600 mg/day) and topical capsaicin (0.075% q.d.s.).
CONCLUSIONS:
Evidence of efficacy exists only for capsaicin 8%, smoked cannabis and rhNGF. However, rhNGFis clinically unavailable and smoked cannabis cannot be recommended as routine therapy. Evaluation of novel management strategies for painful HIV-SN is urgently needed.





























